Introduction
Adenomyosis is defined as the presence or ‘invasion’ of endometrial glands and stroma within the uterine muscle resulting in uterine muscle hypertrophy and hyperplasia and subsequently uterine enlargement [1]. The reported incidence varies from 5 to 70% [2]. This wide variation may be attributed to a lack of defined diagnostic criteria, the study population, or methodological differences in case ascertainment [3]. Adenomyosis is associated with dysmenorrhoea, uterine bleeding disorders, chronic pelvic pain, and dyspareunia as predominant symptoms [4]. Heavy menstrual bleeding and dysmenorrhoea have been the main symptoms, and this could be due to enlarged uterine size, enhanced uterine vascularity, uncoordinated uterine contractions, and altered hormonal (oestrogen and prostaglandin) milieu [4, 5]. It has been demonstrated that the symptoms worsen as the depth and extent of adenomyosis invasion into the myometrium increase, along with the number of adenomyotic foci. Historically, diagnosis was made on histopathological specimen of uterus, but with the introduction of ultrasound and magnetic resonance imaging (MRI), a new dimension to preoperative diagnosis of adenomyosis has been added. Transvaginal ultrasonography (TVS) is the first-line imaging method to diagnose adenomyosis. Three-dimensional (3D) TVS and MRI have also been reported to improve the diagnostic accuracy for adenomyosis [6].
To standardise the reporting of adenomyosis on USG, the Morphological Uterus Sonographic Assessment (MUSA) consensus was published in 2015, which was further modified in 2018 [7]. After this publication, a pilot study reported high inter-reporter disagreement while reporting adenomyosis using MUSA features [8]. So, in 2021, a new Delphi consensus revised the definitions of MUSA features of adenomyosis and classified these into direct (myometrial cysts, hyperechogenic islands, and echogenic sub-endometrial lines and buds) and indirect sonographic signs of adenomyosis (asymmetrical myometrial thickening, fan-shaped shadowing, translesional vascularity, irregular junctional zone, and interrupted junctional zone and globular uterus) [9]. Direct features indicate the presence of ectopic endometrial tissue in the myometrium, and indirect features are those that are secondary to the presence of endometrial tissue in the myometrium. Diagnosis is confirmed if at least one direct feature is present, and the presence of indirect features in the absence of direct features makes the diagnosis uncertain. In such cases, experts recommended evaluation of junctional zone, and its involvement confirms the diagnosis. Exacoustos et al. proposed a study aiming to correlate the type and severity of adenomyosis on ultrasound with the associated symptoms. However, correlation between severity of symptoms and extent of disease could not be established [10]. Another retrospective study including 65 subjects demonstrated no statistically significant difference between patients presenting direct signs and those presenting none for the symptoms considered [11]. None of the prospective study to date has correlated direct and indirect features of adenomyosis with the type and severity of symptoms. So, this prospective study aims to correlate the direct and indirect MUSA features of adenomyosis with severity of clinical symptoms.
Material and methods
This is an observational prospective study conducted at the All India Institute of Medical Sciences Patna, Bihar from April 2023 to March 2024. The study was approved by institute’s Ethics Committee (AIIMS/Pat/IEC/2022/1038).
Inclusion criteria were women aged 18–45 years with regular menstrual cycle, having USG confirmed diagnosis of adenomyosis as per Delphi consensus 2021. Women with a diagnosis of endometriosis, uterine fibroids and polyp, sonographic findings of endometriosis and/or uterine myoma, ovarian cysts or lesions, endometrial hyperplasia or malignancy, coagulopathy, or any medications or hormonal treatment were excluded from the study. According to a study by Pinzauti et al. [12], the prevalence of adenomyosis in the general population attending a gynaecology clinic was 20.9%. Based on this reference, sample size of 264 persons was required to achieve a 95% confidence interval after including a 10% drop out rate. A total of 254 women with ultrasound diagnosis of adenomyosis were included in the study.
A detailed history regarding clinicodemographic data was obtained. The presence of painful menses and heavy menstrual bleeding, chronic pelvic pain (CPP), and any bladder and bowel symptoms was noted. CPP was defined as a painful syndrome originating in one or more pelvic organs, not associated with menstruation, which was intense enough to interfere with the patient’s usual activities and required clinical or surgical treatment, lasting for more than 6 months. Severity of dysmenorrhoea, CPP, dyschezia, or dyspareunia were graded on a visual analogue scale (VAS). VAS score > 7 was considered severe. Severity of menstrual blood loss was assessed using a pictorial blood loss analysis chart (PBAC), and a PBAC score > 100 was considered as heavy menstrual bleeding. Infertility was defined as the inability to conceive after one year of unprotected intercourse.
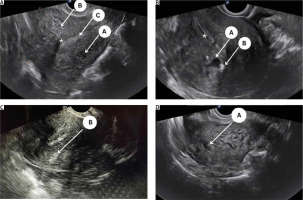
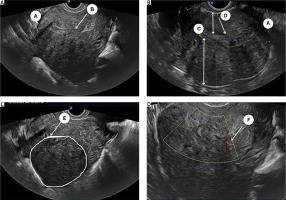
All eligible women underwent a detailed 2D-TVS with grey scale and power Doppler during the secretory phase of the menstrual cycle. All TVS were performed by 2 experienced sonographers (SJ and US) who were blinded to the patient’s clinical symptoms. A thorough evaluation of the pelvis was performed including the uterus (myometrium, endometrium, and junctional zone), bilateral adnexa, anterior and posterior compartment, rectum, bladder, rectosigmoid junction, presence of adhesion (sliding signs), site-specific tenderness, and translesional vascularity. Women with features of endometriosis (endometrioma or deep endometriotic nodule) or myoma were excluded from the study. A diagnosis of adenomyosis was established if one direct MUSA feature or one indirect MUSA feature with involvement of junctional zone was observed [9]. Direct features included were as follows: cysts in the myometrium; hyperechogenic islands; and echogenic subendometrial lines or buds (Fig. 1). Indirect features included those secondary to the presence of endometrial tissue in the myometrium, such as globular uterus, asymmetrical myometrial thickening, fan-shaped shadowing, translesional vascularity, irregular junctional zone, and interrupted junctional zone (Fig. 2). Adenomyosis was defined as diffuse if < 25% of the lesion was surrounded by normal myometrium, provided that > 25% of the entire myometrium was involved, and focal if > 25% of the lesion surrounded by normal myometrium [7].
Statistical analysis
All continuous data were checked for normality using D’Agostino’s K2 test. Continuous data were represented as mean or median with dispersion indices (standard deviation and range). Categorial data were represented as frequency and percentage. The c2 test was used to evaluate the association between clinical symptoms and MUSA features. Stepwise logistic regression analysis with forward selection was performed to find statistically significant direct or indirect features associated with the clinical symptoms. p < 0.5 was considered statistically significant. Data analysis was performed using MedCalc.
Results
The mean age of the studied population was 37 ±7.2 years. Among them, 87.8% were multiparous. Only 8 patients presented with infertility. Most of them had had symptoms for approximately 2 years. The mean PBAC score of the women was 143 ±9.2. The most common presenting symptoms were HMB seen in 42.5%, followed by dysmenorrhoea (34.25%), CPP (29.9%), and dyspareunia (2.7%) (Table 1).
Table 1
Clinicodemographic details of the studied population
Of the 254 analysed cases, 94.4% had at least one direct feature. and the remaining 5.6% had one indirect feature along with JZ involvement. Among direct features, the most common feature noted was myometrial cysts (83.75%). The most common indirect feature noted was asymmetric myometrial thickness (215/254, 84.6%). 86.25% of subjects had diffuse adenomyosis (Table 2).
Table 2
Distribution of direct and indirect morphological uterus sonographic assessment (MUSA) features among the studied population
Clinical symptoms were similar among patients with direct and indirect features except for CPP, which was significantly associated with the presence of direct features of adenomyosis (Table 3).
Table 3
Correlation of symptoms with direct and indirect morphological uterus sonographic assessment (MUSA) features
| Parameters | Number | Direct features | P-value | |
|---|---|---|---|---|
| N = 254 | Present (n = 240) | Absent (n = 14) | ||
| HMB | 108 | 105 | 3 | 0.10 |
| CPP | 83 | 82 | 1 | 0.036 |
| Dysmenorrhoea | 147 | 145 | 2 | 0.05 |
| Dyspareunia | 24 | 24 | 0 | 0.21 |
| Infertility | 8 | 8 | 0 | 0.48 |
| Dysuria | 5 | 5 | 0 | 0.58 |
Table 4 demonstrates the association between individual direct and indirect MUSA features and clinical symptoms. Statistically significant association was noted between HMB and echogenic subendometrial lines and buds, interrupted JZ, asymmetrical myometrial thickening, trans-lesional vascularity, and globular uterus; CPP and myometrial cysts, globular uterus, and Interrupted JZ; dysmenorrhoea and fan-shaped shadowing, myometrial cysts, and hypoechogenic islands; and dyspareunia and asymmetrical myometrial thickening. Older women were frequently associated with interrupted junctional zone and diffuse adenomyosis.
Table 4
Correlation of individual morphological uterus sonographic assessment (MUSA) features of adenomyosis with clinical symptoms
On applying stepwise logistic regression analysis using all MUSA features (indirect and direct both) as independent variables, HMB was found to be significantly associated with asymmetrical myometrial thickening, Interrupted JZ and globular uterus had odds ratios (OR) of 2.84, 2.01, and 4.03, respectively. Chronic pelvic pain was significantly associated with myometrial cyst (OR = 3.07), and dysmenorrhoea with myometrial cyst (OR = 1.13) (Table 5).
Table 5
MUSA features significantly associated with clinical symptoms on logistic regression analysis
Discussion
Clinical symptoms were similar among studied patients with direct and indirect features, except for the CPP, which was significantly associated with the presence of direct feature of adenomyosis. Statistically significant association was noted between HMB and echogenic sub-endometrial lines and buds, Interrupted JZ, asymmetrical myometrial thickening, trans-lesional vascularity, and globular uterus; CPP and myometrial cysts, globular uterus, and Interrupted JZ; dysmenorrhoea and fan-shaped shadowing, myometrial cysts, and hypoechogenic islands; and between dyspareunia and asymmetrical myometrial thickening. Older women were more associated with interrupted junctional zone and diffuse adenomyosis. However, on regression analysis, asymmetrical myometrial thickening (OR = 2.84), interrupted JZ (OR = 2.01), and globular uterus (OR = 4.03) were significantly associated with HMB. Women with myometrial cyst on USG were 3 times more likely to have CPP.
Adenomyosis is identified in 10–70% of hysterectomy specimens and is diagnosed using ultrasound in 20.9% of women attending a general gynaecology clinic [1]. However, in recent years adenomyosis has turned from a histopathological entity into a clinical condition, diagnosed by imaging techniques [7]. In the last decade, transvaginal sonography (TVS) has been described as a diagnostic tool in adenomyosis with a range of sensitivity of 65–81% and specificity of 65–100% [6]. Morphological uterus sonographic assessment classification has standardised the sonographic reporting of adenomyosis, but the association of clinical symptoms with the imaging diagnosis is equally important to devise a management protocol for these patients.
Most of the women with adenomyosis were multiparous (87.8%) in this study, which supports the hypothesis that pregnancy may contribute to adenomyosis by incorporating adenomyotic foci into the myometrium through the invasive action of the trophoblast on myometrial fibres [13]. In the present study, the most common clinical presentation was HMB (42.5%), followed by dysmenorrhoea (34.25%) and CPP (29.9%), which is in alignment with the previous reported literature [10, 14].
The revised criteria indicate that without direct features, an ultrasound cannot conclusively diagnose adenomyosis, which helps prevent overdiagnosis. In this study, 94.4% of symptomatic women were diagnosed with adenomyosis based on the presence of at least one direct feature. Among the direct signs, myometrial cyst was the most common (83.75%) followed by hyperechogenic glands (61.25%), which is consistent with the previous report [10, 15]. Among both the direct and indirect features, asymmetric myometrial thickness and diffuse phenotype were the most commonly observed. This finding is in contrast with the findings of Exacoustos et al., where focal adenomyosis was more common probably because of the association with endometriosis [10]. However, Vannuccini et al. reported a dominance of the diffuse phenotype among an adolescent cohort with HMB and dysmenorrhoea [15]. As the disease progresses and muscular hypertrophy increases, recognising changes becomes easier. Therefore, the presence of ultrasound signs of adenomyosis depends on the population examined.
This study’s findings did show a positive association of CPP with the presence of direct signs. However, a recent retrospective study including 62 patients’ historical data by Biasioli et al. reported no statistically significant difference between patients presenting direct signs and those presenting none for the symptoms considered [11]. This difference might be explained by the fact that myometrial cysts and junctional zone involvement were observed more frequently in the present study.
The present study highlighted that a greater number of ultrasound features were associated with more severe symptoms (HMB, CPP VAS score > 7, and dysmenorrhoea VAS score > 7). Naftalin et al. also demonstrated that the higher the number of adenomyotic features on imaging, the greater the pain score [2]. Marques et al. showed a higher prevalence of sonographic features of adenomyosis in symptomatic patients [14]. A recent study including 53 reproductive-aged women demonstrated a significant positive correlation between the number of sonographic adenomyosis features and both PBAC and NRS scores [16]. After logistic regression analysis, HMB was found to be significantly associated with the presence of asymmetrical myometrial thickening (OR = 2.84), interrupted JZ (2.01), and globular uterus (OR = 4.03) on ultrasound. Myometrial cyst was also associated with severe dysmenorrhoea (OR = 1.13) and CPP (OR = 3.07), which was consistent with the findings of Maldutytė et al., wherein higher PBAC score and NRS score were associated with the presence of globular uterus, interrupted junctional zone, and fan-shaped shadowing [16]. Asymmetric myometrial thickening and interrupted JZ might reflect the increased fibrotic lesion interfering with myometrial contraction, thus resulting in increased blood loss during menses. A recent study investigated the association of stiffness and extent of adenomyotic lesions using transvaginal elastosonography and HMB, wherein a linear relationship was reported between the extent of adenomyosis and volume of menstrual blood loss [17].
Our findings demonstrated significant associations between specific MUSA features and severity of clinical symptoms of adenomyosis. It also highlighted that a greater number of ultrasound features were associated with more severe symptoms (HMB, CPP VAS score > 7, and dysmenorrhoea VAS score > 7). Understanding the correlation between ultrasound features and clinical symptoms allows for a more tailored approach to patient counselling and management. In addition, accurate diagnosis of uterine disease is essential given the risk of misdiagnosed sarcoma during the management of these lesions [18]. This also includes setting realistic expectations for symptom management and potential treatment outcomes.
Strengths and limitations
The main strength of our study is that uniform ultrasonographic criteria by the MUSA 2021 group were used to diagnose adenomyosis. All the TVS were performed by 2 experienced sonographers, and we recruited a large number of subjects (254). Another strength of this study is its prospective design, which makes the findings more reliable. Only a few studies investigated the association of standardised MUSA criteria of adenomyosis with clinical symptoms prospectively; however, the sample sizes of those studies were smaller [15, 16]. We used the PBAC score to quantify the HMB and VAS score, to assess the severity of pain, and hence omitted the assessment bias. A major weakness of our study is the lack of confirmation of the adenomyosis by histology, which is still considered as the gold standard. However, according to a recent meta-analysis, TVS has shown high sensitivity and specificity (84% each) in diagnosing adenomyosis.
Conclusions
Our data show that few sonographic direct and indirect signs of adenomyosis are linearly associated the severity of symptoms. However, diagnosis of adenomyosis should not be based solely on the imaging findings; the patient’s symptomatology should also be considered, especially in the absence of any direct signs.