Introduction
Malignant tumors are often treated by individual surgical resection, radiotherapy systematic oncologic medical treatment or a combination of these methods. Advancements in techniques and content have led to successful results in recent years. However, the risk of local recurrence and distant metastasis still persists. The presence of metastatic disease evidently has an effect on treatment outcomes and overall survival of patients. The lungs are a common site for metastasis of certain systemic malignancies such as breast cancer, colorectal cancer, sarcoma, and head/neck squamous cell carcinomas. Due to this common clinical situation, there has been ongoing debate about the most effective approach and purpose of surgery for solitary lung metastasis in frequent clinical cases since the 1970s. However, there are no randomized prospective studies comparing surgical resection with other treatment methods. It is widely understood that some patients with a pulmonary metastasis, whose primary tumor shows no signs of activity (no local recurrence or any either metastatic site), can benefit from undergoing pulmonary metastasectomy [1]. The current surgical referral is based on a publication from the International Lung Metastasectomy Unit [2] where the 5-year follow-up after pulmonary metastasectomy survival rate was in the range 20–40% [3]. A general rule of thumb is to ensure that the disease is being managed at the local level and that there are no indications of its spread across the body.
As minimally invasive surgery is becoming more commonly employed, there is a debate concerning the uses of the minimally invasive approach, video-assisted thoracoscopic surgery (VATS) and the classical open (thoracotomy) approach for metastasectomy cases.
Open surgery via thoracotomy is recommended due to the advantage that the lung can be palpated manually by the surgeon, making it possible to detect additional lesions, undiagnosed by radiological imaging [4]. Due to the advances IT technology in the last decade, the need for this has gradually decreased. The potential benefits of VATS for postoperative pain management and respiratory functioning have been demonstrated. Additionally, studies have shown that VATS allows for re-metastasectomy with reduced morbidity and comparable outcomes to open surgery [5–9].
In our opinion, resection for isolated lung metastases is the most reliable method for diagnosis. To maximize the chances of survival, resection using video-assisted thoracic surgery VATS is recommended. Here we report our findings on the use of minimally invasive surgery (VATS) for pulmonary metastasectomy, with a particular focus on identifying the most suitable candidates for the surgery. Our inclusion criteria were as follows: the primary tumor should be treated with no signs of local recurrence (viable tissue) and isolated organ (lung) metastasis. According to the standard procedure with all lung surgery patients, adequate respiratory capacity measures are mandatory. Our exclusion criteria were as follows: insufficient respiratory function, multi-organ metastasis, local recurrence, potential other high medical risks due to which the patient is unwilling to undergo surgery. We have considered the use of VATS due to its less invasive nature and the potential for better pain management. Conducting a study to verify the positive outcomes of surgery in this specific patient population, while considering relevant confounders, might aid in the process of selecting patients.
Aim
Our aim is to report our findings on the use of VATS for pulmonary metastasectomy, with a focus on identifying suitable candidates.
Material and methods
Patients
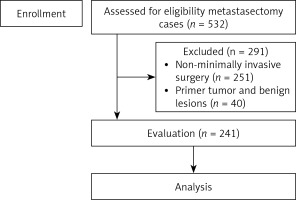
Between August 2010 and 2023 a total of 532 pulmonary metastasectomy procedures were performed in our institution. Metastasectomy was performed with VATS for 281 of those patients. The pathology results showed that 40 patients had a primary tumor and benign lesions, so they were not included (Figure 1). A total of 241 patients who met the inclusion criteria made up the study group and their data were reviewed retrospectively.
All patients discussed at the oncology council underwent positron emission tomography/computed tomography (PET-CT) scanning to confirm the presence of lung metastasis without any additional metastases outside the thoracic region, and it was determined that the primary tumor was under control. Pre-operative respiratory function tests showed the available lung function for pulmonary resection including lobectomy. If the patient had a suspected lesion for primarily lung cancer, preoperative diagnosis by fine needle aspiration was planned.
All patients underwent a multidetector thin-section thorax CT scan days before surgery (1–7 days). All imaging analyses were supervised by both a senior radiologist and surgeons.
Surgical procedure
Patients were placed in a lateral decubitus position after double lumen intubation. Depending on the preference of the surgical groups, one or two incision ports were placed in the intercostal spaces in the 5th and 7th intercostal spaces. By using surgical instruments, a general view was achieved. In addition to focusing on the specific lesion identified through CT findings, a broader examination was also conducted. Once the lesions had been clearly identified, the lung was then grasped at the site of the lesion and a compressive clamp was placed below the lung lesion along the anticipated surgical margin line. Once the surgical margin distance appeared to be acceptable (2–3 cm), a parenchymal endostapler was placed and wedge resection was completed. The integrity of the stapler line and margin was evaluated, and the stapling process was continued below the parenchymal resection line.
The most commonly used tissue preservation method is wedge resection. However, it is necessary to ensure that the surgical margins are safe and clean while preserving the parenchyma for additional surgery, therefore potentially accepting the possibility of lobectomy. This may be disadvantageous in terms of providing the patient with more cardiopulmonary reserve to withstand subsequent treatments in case of the recurrence of the disease and preserving the patient’s quality of life. When conversion to open surgery is necessary, it should be carried out without hesitation.
Statistical analysis
Mean, standard deviation, median, lowest, highest, frequency and ratio values were used in the descriptive statistics of the data. Cox regression (univariate-multivariate) and Kaplan-Meier curves were used for the purpose of survival analysis. The SPSS 28.0 program was used in the analyses.
Results
Of 241 patients, 133 (55.2%) were male and 108 (44.8%) were female (Table I). The median patient age was 56 (5–79) years. Metastasectomy via VATS was performed in 131 patients with a single lesion while 110 patients underwent metastasectomy for multiple lesions. The histopathological distribution of the primary tumor and other clinical characteristics of the patients are shown in Table I.
Table I
Clinical characteristics of overall patients
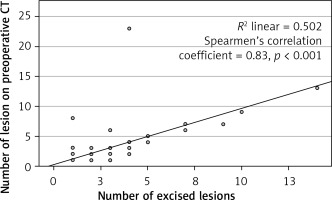
In the final pathology, it was found that 147 (61.0%) patients had single lesions, whereas 94 (39.0%) patients had numerous lesions. The median number of lesions found on preoperative thorax CT scans was 1 (1–23) and the median number of excised lesions was 1 (1–13). When the number of preoperative CT lesions and the number of surgically removed lesions were examined, it was observed that there was no significant difference (p > 0.001). There was a significant correlation between the number of lesions excised by VATS metastasectomy and the number of lesions reported on preoperative images (n = 418/455) (Spearman’s correlation coefficient 0.83; p < 0.001) (Figure 2).
The following factors were considered and calculated: age, gender, primary malignancy site, procedure side, treated lobe, preoperative chemotherapy and/or radiotherapy, surgical procedure, lymph node excision and presence of metastasis, occurrence of complications, number of metastasectomies, time between metastasectomies, number of operations, postoperative length of stay (Table II).
Table II
Results
In the univariate model, age, gender, primary malignancy site, procedure side, procedure lobe, preoperative chemotherapy and/or radiotherapy, surgical procedure, lymph node excision, lymph node metastasis, complications, number of metastasectomies, time between metastasectomies, number of operations and postoperative length of stay were not observed to have a significant effect on survival time (p > 0.05) (Table III).
Table III
Factors effecting the survival
In the univariate model, it was observed that tumor histology, number of preoperative CT lesions, and number of excised lesions had a significant effect on survival time after surgery (p < 0.05) (Table III).
In the multivariate model, it was observed that the number of lesions removed had a significant independent effect on survival time (p < 0.05) (Table III).
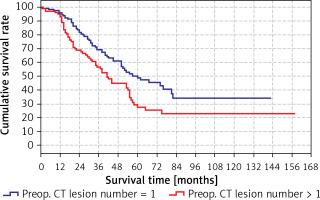
The predicted survival time in the group with preoperative CT lesion count > 1 (41 months) was significantly (p < 0.05) lower than the group with preoperative CT lesion count 1 (60 months) (Tables IV, V). Survival rates according to preoperative lesion numbers are shown in Table V and Figure 3.
Table IV
Effect of number of preoperative ct lesions on survival
| Parameter | Survival time [months] | 95% CI | P-value | |
|---|---|---|---|---|
| Preop. CT | Lesion number = 1 | 60.0 | 41.1–78.9 | 0.007 |
| Lesion number > 1 | 41.0 | 33.8–48.2 | ||
| Total | 53.0 | 45.6–60.4 | ||
Table V
Cumulative survival rate
| Year | Cumulative survival rate | ||
|---|---|---|---|
| Total | Preop. CT lesion number | ||
| 1 | > 1 | ||
| 1 | 95.7% | 96.2% | 95.1% |
| 2 | 77.0% | 83.4% | 68.8% |
| 3 | 63.6% | 69.2% | 56.5% |
| 4 | 54.0% | 60.9% | 44.7% |
| 5 | 41.0% | 50.1% | 29.1% |
| 10 | 29.4% | 34.2% | 22.8% |
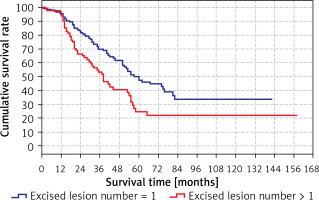
The predicted survival time in the group with the number of excised lesions > 1 (38 months) was significantly (p < 0.05) lower than in the group with the number of excised lesions > 1 (60 months) (Tables VI, VII). Survival rates according to the number of lesions excised are shown in the Table VII and Figure 4.
Table VI
Survival of one or more excised lesions
| Variable | Survival time [months] | 95% CI | P-value | |
|---|---|---|---|---|
| Excised | Lesion number = 1 | 60.0 | 46.9–73.1 | 0.002 |
| Lesion number > 1 | 38.0 | 29.1–46.9 | ||
| Total | 53.0 | 45.6–60.4 | ||
Table VII
Cumulative survival rate
| Year | Cumulative survival rate | ||
|---|---|---|---|
| Total | Number of lesions excised | ||
| 1 | > 1 | ||
| 1 | 95.7% | 95.2% | 96.5% |
| 2 | 77.0% | 83.3% | 66.4% |
| 3 | 63.6% | 69.8% | 53.2% |
| 4 | 54.0% | 61.5% | 40.5% |
| 5 | 41.0% | 49.9% | 24.5% |
| 10 | 29.4% | 33.7% | 21.6% |
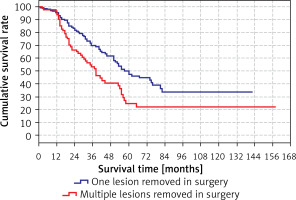
The survival rate was significantly (p < 0.05) lower in the group with multiple lesions removed during surgery (38 months) than in the group with only one lesion removed during surgery (60 months) (Tables VIII, IX).
Table VIII
Survival according to single and multiple lesions
| Variable | Survival time [months] | 95% CI | P-value | |
|---|---|---|---|---|
| Number of excised lesion | One | 60.0 | 46.9–73.1 | 0.002 |
| Multiple | 38.0 | 29.1–46.9 | ||
| Total | 53.0 | 45.6–60.4 | ||
Table IX
Cumulative survival rate
| Year | Cumulative survival rate | ||
|---|---|---|---|
| Total | Lesion removed in surgery | ||
| One | Multiple | ||
| 1 | 95.7% | 95.2% | 96.5% |
| 2 | 77.0% | 83.3% | 66.4% |
| 3 | 63.6% | 69.8% | 53.2% |
| 4 | 54.0% | 61.5% | 40.5% |
| 5 | 41.0% | 49.9% | 24.5% |
| 10 | 29.4% | 33.7% | 21.6% |
Survival rates according to the lesions removed in surgery are shown in Table IX and Figure 5.
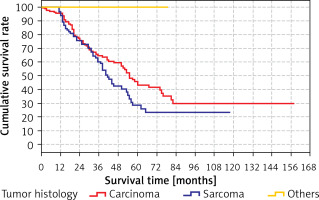
There was no significant (p > 0.05) difference between the predicted survival time of the group with tumor histology as carcinoma (41.4 months) and the groups with tumor histology as sarcoma (55.5 months). The predicted survival time in the group with other tumor histology (79 months) was significantly (p < 0.05) higher than in the groups with tumor histology carcinoma (41.4 months) and sarcoma (55.5 months) (Table X). Kaplan-Meier analysis did identify any differences for these variables.
Table X
Survival by histology
| Tumor histology | Survival time [months] | P-value |
|---|---|---|
| Carcinoma | 41.4 | 0.011 |
| Sarcoma | 55.5 |
Survival rates according to the tumor histology are shown in Tables X, XI and Figure 6.
Table XI
Cumulative survival rate
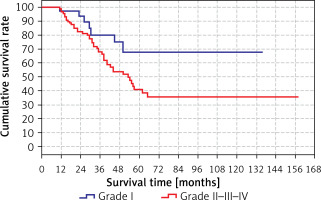
The difference was not significant when the grades were analyzed one by one, but it was significant when they were divided into two groups (grade I and grades II, III, IV). The survival time (80.1 months) in the grade II/III/IV group was significantly (p < 0.05) shorter than the grade I group. The results are shown in Tables XII, XIII (in Table XII, the total time is different from the other results because the analysis was performed only in the grade group) and Figure 7.
Table XII
Survival rate by grade
| Variable | Survial rate [months] | 95% CI | P-value | |
|---|---|---|---|---|
| Grade | I | 103.1 | 82.1–124.0 | 0.032 |
| II–III–IV | 80.1 | 65.0–95.3 | ||
| Total | 89.3 | 75.8–102.7 | ||
Discussion
Although the primary malignant tumor is treated locally with surgery and radiotherapy, treatment practices for systemic metastases continue to be a topic of debate. Lung metastases of primary malignant tumors require exceptional evaluation among systemic metastases. It has been reported that the number of nodules detected in secondary pulmonary neoplasms, particularly in thorax CT, is 50% lower than the number of nodules detected during surgery [10, 11]. In our study, the median number of lesions found on preoperative thorax CT scans was 1 (between 1 and 23) and the median number of excised lesions was 1 (between 1 and 13). There was a significant correlation between the number of lesions excised by metastasectomies via VATS and the number of lesions reported on preoperative images (n = 418/455) (Spearman’s correlation coefficient 0.83; p < 0.001). It is thought that the reason is the use of newly developed technological devices with thin incisions and three-dimensional imaging in axial, coronal and sagittal planes. Additionally, this approach will prevent unnecessary re-metastasectomy. While performing surgery, it is essential to preserve the intact parenchyma as much as possible, and to ensure that the surgical margins are safe and free from tumors. In cases where the parenchyma is preserved but the surgical margins are not tumor-negative, a metastasectomy will not contribute to survival [12, 13]. For this reason, anatomic resection was performed in cases where it was considered that tumor-negative surgical margins would not be possible with other types of resection (lobectomy) in centrally located lesions. Anatomic resection was performed in 12 (4.97%) cases due to multiple metastases and these rates are corroborated by the literature [14].
In our study, the mean follow-up time was calculated as 58.6 months and the cumulative 5-year survival rate was found to be 43.8%, similar to the rates reported in the literature [10, 12–14]. This is especially related to the high accuracy rate of nodules detected by new technology imaging methods and the excision of additional nodules that we detect preoperatively. Recurrences may occur after metastasectomy, especially in sarcomas and melanomas. In these cases, if metastasectomy criteria are applicable, re-metastasectomies may even be performed on the same side. In our study, re-metastasectomy was performed in 42 (17.4%) cases and contributed to survival times by an average of 45 months. Studies on typical open surgical procedures have indicated a 5-year survival rate of 54% and a recurrence rate of 30%. It is noteworthy that the recurrences we reported were most common in the contralateral lung and that ipsilateral recurrences could be operated on with VATS in the majority of cases; this adds some weight to the notion that most patients with solitary metastases will have a high proportion of lung tissue and will benefit from a minimally invasive surgical approach [15]. The predicted survival time in the group with other tumor histology (79 months) was significantly (p < 0.05) higher than in the groups with tumor histology carcinoma (41.4 months) and sarcoma (55.5 months). Our survival rates in cases are higher than those in the literature [16]. Imaging methods allow detailed imaging of the lung parenchyma since it is located within a limited area in the rib cage, thus facilitating early detection of metastases.
In the sarcoma group, the 5-year survival was 35% and the overall survival of metastasectomy was calculated as 37.42 months. Nevala et al. reported the 22-month overall survival after pulmonary metastasectomy for sarcomas, and a higher survival rate was found in our study [17]. In our study, re metastasectomies were performed mostly in the osteosarcoma group. The synovial sarcoma group had the highest number of lesions excised at one time, with 14 lesions.
Since not all metastatic nodules may be detected radiologically, intraoperative lung inspection and evaluation should be carried out carefully. The presence of additional nodules should be investigated by digital and instrument-based inspection. In this regard, the main factors determining survival are thought to be tumor histopathology and the extent of the disease. Surgical approaches to pulmonary metastasectomy should be determined according to the number and location of the nodules. Since it is essential to remove all nodules, two-session interventions may be performed if necessary. Nodules whose presence is revealed radiologically should also be revealed during the operation; otherwise, they cannot be detected radiologically.
Conclusions
Metastases are a sign of rapid disease progression along with uncontrolled tumor growth [18]. However, isolated lung metastases follow favorable tumor biology. These patients are more suitable for local and local-systemic treatment applications than cases with multiple organ metastases. In all branches of expertise related to malignancy, imaging methods to evaluate the lungs should be used in cases with primary controlled tumors, and when the possibility of isolated pulmonary metastasis is detected, the opinion of the thoracic surgeon should be obtained and cases that meet the metastasectomy criteria should be referred to surgery. We have found that utilization of VATS in metastasectomies is safe and yields a similar survival rate. Since it is essential to remove all nodules, two-session interventions can be performed if necessary; VATS provides an advantage in this case. Nodules that are detected radiologically should also be revealed during the operation. Additionally, keeping in mind that there may be nodules that cannot be detected radiologically, careful detection should be performed and all detected nodules should be removed. The best prognosis after metastasectomy is provided in cases with a single nodule. Grade is also an important prognostic factor affecting survival, particularly for grade 1 tumor. In secondary pulmonary neoplasms, gender does not affect the contribution of metastasectomy to survival. The histopathological type of the primary tumor is also a significant prognostic factor affecting survival after pulmonary metastasectomy in secondary pulmonary neoplasms, particularly for sarcoma and carcinoma.