Introduction
Hepatocellular carcinoma (HCC) is the most common primary hepatic tumor in adults and is responsible for the third-highest number of cancer-related deaths worldwide, according to the World Health Organization (WHO) [1]. Multiple targeted therapies are available for HCC depending on the category of classification that patients are diagnosed with [1]. The Child-Pugh score assesses the prognosis and severity of liver disease, mainly cirrhosis, along with the expected survival rate and need for liver transplantation. Tyrosine kinase inhibitors, such as lenvatinib, are recommended in patients with chronic liver disease with Child-Pugh class A and preserved liver function [2]. Another classification system is the Barcelona Clinic Liver Cancer (BCLC) that assesses the progression of HCC, liver function, as well as performance status. Lenvatinib has shown efficacy in patients with BCLC stage B or C refractory to transcatheter arterial chemoembolization (TACE) [3].
During the past decade, the main first-line medication prescribed to patients with unresectable HCC has been sorafenib. In 2018, the U.S. Food and Drug Administration (FDA) approved lenvatinib as a first-line treatment for unresectable HCC due to several positive phase III clinical trials [4, 5]. Previously, the FDA had approved lenvatinib for use in radioactive iodinerefractory follicular and papillary differentiated thyroid carcinoma (DTC), advanced endometrial cancer, and as a combination therapy with everolimus in renal cell carcinoma (RCC). Currently, lenvatinib applications in the treatment of urothelial carcinoma, squamous cell carcinoma of the head and neck, and non-small cell lung cancer are in phase III of clinical trials evaluating its safety and efficacy [4, 5].
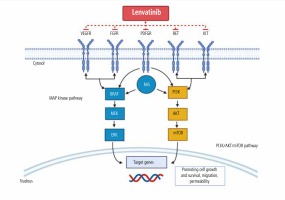
Lenvatinib is a multi-targeted tyrosine kinase inhibitor (TKI) aimed at inhibiting vascular endothelial growth factor receptors (VEGFR) 1-3, fibroblast growth factor receptor (FGFR) 1-4, C-KIT, RET protooncogene, and platelet-derived growth factor receptor α (PDGFR-α). Several of these receptor proteins play a major role in cancerous signaling pathways [6, 7]. Specifically, these receptors enhance the MAP kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway found in cancer cells and induce tumor angiogenesis and growth, as illustrated in Figure 1 [6-8]. TK1 multi-receptor inhibitors inhibit migration and invasion of tumor cells into normal functioning cells [6, 7].
Fig. 1
The two major signaling pathways: MAP kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) targeted by lenvatinib [8]
The results of many clinical trials on the safety and efficacy of lenvatinib in patients with solid tumors, such as thyroid, colorectal, lung, liver, RCC, and head and neck, have shown significant improvement, despite many adverse effects [9]. Patients who experience adverse effects, such as hypertension and proteinuria, have shown a higher efficacy level from lenvatinib [9]. This review discusses the clinical implication and treatment outcomes for patients with unresectable HCC treated with lenvatinib as first-line therapy.
Methodology
The authors searched for over 100 articles and used 39 peer-reviewed publications related to the topic of lenvatinib as a targeted therapy for advanced/unresectable hepatocellular carcinoma. This systematic review is centered on the mechanism of therapy, first-line indications, interventions, efficacy, and real world analysis of lenvatinib use worldwide. A literature search using case reports, meta-analysis, cohort studies, retrospective studies, and narrative reviews from PubMed, PubMed Central, and Google Scholar was analyzed and interpreted.
Staging of hepatocellular carcinoma
Child-Pugh classification score
The Child-Pugh classification score measures the level of damage to the liver due to cirrhosis [10, 11]. The score consists of five categories, total bilirubin (µmol/l or mg/dl), serum albumin (g/dl), either prothrombin time or international normalized ratio (INR), ascites, and hepatic encephalopathy, each of which receives 1-3 points, with 3 being the most severe form. The Child-Pugh score obtained can be divided into class A with 5-6 total points and a 100% first-year survival rate, class B with 7-9 total points and an 80% first-year survival rate, and class C with 10-15 total points and a 45% first-year survival rate [10, 11].
Barcelona Clinic Liver Cancer classification score
The BCLC staging system is divided into five stages and incorporates the Eastern Cooperative Oncology Group (ECOG) scale to check for performance status (PS) [12, 13]. The ECOG scale ranges from 0 points when the patient is fully active with similar function before the diagnosis of liver disease to 4 points when the patient is bedridden or needs the use of a wheelchair along with complete care. With the PS and the Child-Pugh scores in mind, the BCLC can strengthen the overall prognosis and survival rate. The BCLC score begins with the very early stage 0 that consists of only one tumor less than 2 cm, with an ECOG score of 0 and Child-Pugh class A. The BCLC stage A consists of less than 3 tumors smaller than 3 cm and stage B consists of more than 3 tumors with at least one larger than 3 cm. Both BCLC stage A and B present with an ECOG score of 0 and Child-Pugh class A or B [12, 13]. BCLC stage C is an advanced form of cancer that has spread to blood vessels and surrounding structures with an ECOG score of 1 or 2 and Child-Pugh class A or B. Lastly, stage D in the BCLC scoring system refers to end-stage HCC that has metastasized to various parts of the body with no response to treatment and Child-Pugh class C and an ECOG score of 3 or 4 [12, 13].
Lenvatinib as first-line therapy for advanced hepatocellular carcinoma
The heterogeneity of HCC makes the disease vary immensely among patients in tumor histology, tumor size, metastases, and the level of cirrhosis present [14]. The prognosis in patients with stage one HCC and minimal cirrhosis is far superior to advanced HCC as surgical resection or liver transplantation may be considered for early stage HCC. With progression of disease other therapeutic modalities are available, such as radiofrequency ablation (RFA), TACE, and several systemic therapies [14]. Radiofrequency ablation uses high-frequency radio waves to create very high temperatures that will burn off the tumor [15]. Radiofrequency ablation is preferred for smaller tumors (less than 5 cm) and enhances survival due to it being less invasive than surgery. The TACE procedure, which injects antitumor and embolic agents to inhibit vascular supply to the tumor successfully has shown an increased survival benefit in patients with tumors smaller than 8 cm and normal liver function. The treatment options offered to patients depend on the stage of cancer and their ability to withstand surgery. Additionally, liver transplantation, if available, is an option for some patients who can tolerate invasive surgery to increase survival outcomes [15].
Patients with advanced HCC that is refractory to TACE or surgery can receive systemic treatment. The anti-kinase inhibitor sorafenib was approved in 2006 as the standard, first-line therapy for advanced HCC. The randomized controlled, double-blind, placebo-controlled study carried out by Sorafenib HCC Assessment Protocol in 2007 displayed significant superiority of sorafenib to other molecular targeted therapies [16]. Patients in this trial with advanced HCC had drastically increased overall survival rates compared to placebo. Similarly, global clinical trials compared sorafenib to other therapies, such as sunitinib, brivanib, and linifanib. No other therapy was observed to increase the overall survival benefit compared to sorafenib, which became the mainstay of advanced HCC treatment for over ten years [16].
REFLECT clinical trial for lenvatinib use in advanced hepatocellular carcinoma
Lenvatinib was subjected to clinical trials to assess it as first-line therapy for advanced HCC specifically. In the randomized, phase III clinical trial REFLECT, lenvatinib was compared to sorafenib for its efficacy against unresectable HCC (Child-Pugh class A only). The REFLECT trial consisted of 954 patients with specific inclusion criteria of unresectable HCC diagnosis with smaller tumors and minimal cirrhosis. Patients were also selected based on no prior history of systemic therapy. Approximately half the patients were randomly assigned to lenvatinib therapy (n = 478), with doses of 8 mg given to patients weighing less than 60 kg and 12 mg given to patients weighing more than 60 kg. Similarly, 476 patients were given sorafenib 400 mg twice a day [17, 18]. The study’s primary defining factor was overall survival rates, and secondary factors were progression-free survival benefit and objective response rate. Moreover, out of all the patients included in this trial, 217 patients had hepatitis C, and 479 patients had hepatitis B. The primary endpoint for this trial showed a median overall survival rate of 13.6 months with lenvatinib therapy vs. 12.3 months with sorafenib therapy. Lenvatinib did not have a statistically significant benefit over sorafenib in terms of overall survival, HR = 0.92 (95% CI: 0.79-1.06, p < 0.001) [17, 18]. However, the trial showed significant improvement for the secondary endpoints used in this study [17, 18]. The progression-free survival benefit was observed to double the median for lenvatinib therapy of 7.3 months compared to sorafenib therapy with 3.6 months, HR = 0.65 (95% CI: 0.56-0.77, p < 0.001) [17, 18]. The disease’s overall risk for progression or death was reported as 36% for lenvatinib compared to sorafenib therapy. The other secondary endpoint, objective response rate, was significantly superior with triple the responses for lenvatinib compared to sorafenib therapy. The patients on lenvatinib therapy had a 41% response rate (95% CI: 35-45%) and 12% with sorafenib (95% CI: 10-16%) [17, 18].
A common side effect of treatment with lenvatinib is drug-induced hypertension. The increase in blood pressure is more likely to occur in patients older than 75 receiving treatment. During the phase III REFLECT trials, the adverse effects of lenvatinib therapy accounted for greater than 20% of adverse reactions. The most common adverse events reported were hypertension (45%), fatigue (44%), diarrhea (39%), decreased appetite (34%), arthralgia/myalgia (31%), decreased weight (31%), abdominal pain (30%), palmar-plantar erythrodysesthesia syndrome (27%), proteinuria (26%), dysphonia (24%), hemorrhagic events (23%), hypothyroidism (21%), and/or nausea (20%). Serious adverse events occurred in more than 2% of patients in the REFLECT trial presenting with hepatic encephalopathy (5%), hepatic failure (3%), ascites (3%), and/or decreased appetite (2%). The REFLECT trial did not display a statistically significant decline in adverse effects for either lenvatinib or sorafenib therapy [19, 20]. However, dose reduction and treatment discontinuation of lenvatinib therapy due to adverse effects were observed in 62% and 20% of patients, respectively. Adverse effects should prompt supportive management, dosing modifications, and discontinuation of therapy if necessary [19, 20]. Subjects with liver impairments also require dosing modification when receiving lenvatinib as it is metabolized hepatically by the CYP450 isoform CYP3A and aldehyde oxidase and cleared renally as urine and feces [19]. The agent has a half-life of twenty-eight hours, and subjects with severe renal dysfunction characterized by creatinine clearance (CLcr) 15-29 ml/min are subject to diminished drug clearance, further increasing plasma concentrations of lenvatinib and susceptibility to adverse effects [19].
Lenvatinib use in unresectable hepatocellular carcinoma for Child-Pugh class B patients
The success of the REFLECT trial for lenvatinib usage in unresectable HCC for patients with Child-Pugh A liver function led to approval for management of these patients. The possibility of lenvatinib use in Child-Pugh class B function needs additional research and was examined in the post hoc analysis of the REFLECT trial presented at the 2021 Gastrointestinal Cancers Symposium [21]. The post hoc analysis compared patients in the REFLECT trial on lenvatinib and sorafenib for unresectable HCC who began with Child-Pugh class A and declined to Child-Pugh class B within 8 weeks to patients who sustained their Child-Pugh class A liver function during the 8 weeks. The analysis compared the objective response rate (ORR), progression-free survival (PFS), and overall survival (OS) for both groups of patients [21]. About 60 patients who were randomly given lenvatinib therapy in the REFLECT trial progressed to Child-Pugh class B within 8 weeks of treatment, whereas 413 patients maintained their Child-Pugh class A liver function within the 8 weeks of therapy. For the patients on lenvatinib therapy whose liver function declined to Child-Pugh class B in 8 weeks, the median OS was 6.8 months, PFS was 3.7 months and the ORR was 28.3% with a median of 1.9 months during treatment. For the patients on sorafenib therapy whose liver function declined to Child-Pugh class B in 8 weeks, the median OS was 4.5 months, PFS was 0.5 months and the ORR was 8.5% with a median of 1.9 months during treatment. The patients who maintained their liver function of Child-Pugh class A within 8 weeks reported an ORR of 42.9% for those receiving lenvatinib therapy and an ORR of 12.9% for those receiving sorafenib therapy. The percentage of adverse effects noted in patients who progressed to Child-Pugh class B function and who maintained their Child-Pugh class A function was 71.7% and 54.7%, respectively. The percentage of treatment discontinuation noted in patients who progressed to Child-Pugh class B function and who maintained their Child-Pugh class A function was 18.3% and 7.5%, respectively. The post hoc analysis indicated that efficacy of lenvatinib therapy for Child-Pugh class A and Child-Pugh class B liver function showed limited results for a thorough comparison. However, the analysis suggested that patients on lenvatinib therapy for unresectable HCC who show a decline of liver function to Child-Pugh class B within 8 weeks should continue with lenvatinib treatment [21].
Lenvatinib drug-drug interactions
Drug interaction of CYP3A4 and the P-glycoprotein (P-gp) inhibitor ketoconazole co-administered with lenvatinib was tested in a phase I, randomized two-period, crossover clinical trial. 400 mg of ketoconazole for 18 days increased the AUC and Cmax of a single dose of lenvatinib on the fifth day by 15% and 19%, respectively. A phase I, a single-dose sequential study conducted on 15 healthy participants also reported the effects of the P-gp inhibitor rifampicin and its drug interaction with lenvatinib. 600 mg of rifampicin increased AUC and Cmax of a single 24 mg dose of lenvatinib by 31% and 33%, respectively. Both studies concluded that the changes in lenvatinib concentration with co-administration with either agent did not result in clinical implications [19, 22].
Drug interactions with lenvatinib were also observed in patients taking acyclovir and acamprosate, who can present with decreased excretion of the drug. Patients taking acetaminophen with lenvatinib can present with an increased serum concentration of acetaminophen. Lastly, the risk of QTc prolongation can be increased in patients taking epinephrine/adenosine in combination with lenvatinib. Patients taking lenvatinib are also not recommended to consume any hypertensive herbs, such as bayberry, blue cohosh, cayenne, ephedra, and licorice, as hypertension is a common side effect of lenvatinib [19, 22].
Evaluation criteria for response to lenvatinib therapy
The response to lenvatinib therapy is evaluated by physicians treating patients with unresectable HCC with lenvatinib therapy with the Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST (mRECIST) criteria [23, 24]. The criteria outline the use of triphasic scanning methods with computed tomography (CT) or magnetic resonance imaging (MRI) evaluating the liver tumors only once during the first 8 weeks of therapy and every 8 weeks afterwards. The decision to continue therapy was based on the imaging results after the first 8 weeks of therapy, called the best response assessment. According to the mRECIST criteria, a complete versus partial response to lenvatinib therapy is classified as total resolution of disease versus at least 30% resolution of tumors calculated as the sum of the longest diameters, respectively [23, 24]. The assessment that shows progression of the disease is the appearance of new tumors or an increase of approximately 20% or more in the size of previous tumors. Stable disease is not classified as either complete, partial or progressive response. Furthermore, physicians should base the efficacy of lenvatinib on whether or not patients meet the criteria for the REFLECT trial [23, 24].
Literature review evaluating lenvatinib use for advanced hepatocellular carcinoma
A multicenter, retrospective study by Cheon et al. analyzed 92 Korean patients from September 2018 to January 2020 on lenvatinib for advanced HCC [25]. In the study 67 patients received lenvatinib as a first-line therapy, 14 patients received lenvatinib as a second-line therapy, and 11 patients received lenvatinib as a third-line or later-line therapy. Furthermore, 29 patients received surgical resection, 9 patients received RFA, and 54 patients received TACE before starting the trial. The results of the trial results showed significant efficacy and response to lenvatinib, with 60 patients maintaining a stable, non-progressive disease course and only 19 patients exhibiting variable progression of disease throughout the study. The results showed that the patients receiving lenvatinib as a first-line therapy did not differ from patients with second, third, or later-line therapy in terms of progression-free survival or overall survival. However, patients receiving lenvatinib as a first-line therapy did have a significantly higher objective response rate and compliance than patients with second, third, or later-line therapy [25]. Patients receiving lenvatinib with a lesser extent of disease and smaller tumor size were observed to have significantly higher overall survival rate, progression-free disease periods, and objective response rate. Patients who fell into the category of extensive tumor burden in the liver with greater tumor sizes, portal vein, bile duct invasion, and advanced cirrhosis had drastically poorer overall survival, lower progression-free survival, and a lower objective response rate. The overall safety profile of lenvatinib in all 92 patients showed favorable outcomes with some adverse effects. The most common adverse effects noted were elevated aspartate aminotransferase (AST) in 48 patients, fatigue in 36 patients, hyperbilirubinemia in 24 patients, and thrombocytopenia in 24 patients. The investigators concluded that the use of lenvatinib was far superior compared to other systemic therapies in terms of efficacy and safety profile in patients with advanced HCC with smaller tumors [25]. Patients with advanced HCC with larger tumor sizes and extensive cirrhosis have poorer outcomes with lenvatinib, which is due to the severity of the disease [25]. The most significant improvement was observed as progression-free survival in most patients with a longer duration of therapy [25].
A retrospective cohort study conducted by Singal et al. consisting of 233 ethnically diverse patients (52.4% Caucasian and 24.5% African American), with a median age of 62.9 years of age, in academic centers and clinical settings across the United States was performed to evaluate the therapeutic effectiveness of lenvatinib as monotherapy for unresectable hepatocellular carcinoma [26]. 67.8% of the patients studied were male. Individual results were taken from the patients’ medical files. 100% of the patients in this study had preexisting unresectable hepatocellular carcinoma. Assessment of each patient’s health condition included the cause of unresectable HCC, the severity of cirrhosis via the Child-Pugh score, Charlson Comorbidity Index, ECOG PS, and monitoring the tumor stage via Union for International Cancer Control and BCLC. The majority of these patients had Barcelona Clinic Liver Cancer stage B or C disease [26]. 11.2% of the patients were diagnosed with BCLC stage A, 28.8% were diagnosed with BCLC stage B disease, 43.8% had BCLC stage C disease, and 8.2% of the patients had BCLC stage D. Additionally, in 8.2% of the patients the BCLC stage was not known; BCLC staging was identified during the beginning of the administration of lenvatinib. The underlying causes of the diagnoses were hepatitis C, alcoholic liver disease, hepatitis B, nonalcoholic steatohepatitis, respectively; 36.1% of the patients had unresectable HCC due to hepatitis C, 28.3% due to alcoholic liver disease, 15.5% due to hepatitis B, and 13.7% due to nonalcoholic steatohepatitis. In this study, cirrhosis was a common occurrence respective to unresectable HCC: 44.6% Child-Pugh class A, 39.1% Child-Pugh class B, 7.3% Child-Pugh class C. Child-Pugh class was not known in 9% of the patients [26]. Invasion of the portal vein was noted in 18.5% of the patients; of the 18.5% of the patients with portal vein invasion, 7.0% had invasion of the main portal vein. Before the onset of lenvatinib monotherapy, 20.2% of the patients had a history of medical procedures such as transarterial chemoembolization and radiofrequency ablation; of the 20.2%, 10.7% of the patients underwent transarterial chemoembolization, and 8.2% underwent radiofrequency ablation. Patients were administered doses that ranged from < 12 mg to 24 mg. The median dose at the start of lenvatinib treatment was 12 mg; in fact, dosages ranged from < 12 mg, 12 mg, 14 mg, 18 mg, 20 mg, and 24 mg [26]. The preferred dose was dependent on patient weight and disease severity. 52.9% of the patients who weighed under 60 kg were commonly administered an 8 mg dose of lenvatinib. 44.9% of the patients who weighed over 60 kg were commonly administered a 12 mg dose of lenvatinib. Additionally, 50% of the patients who weighed under 60 kg with Child-Pugh class A were administered 4 mg of lenvatinib; nonetheless, 47.6% of the patients who weighed over 60 kg with Child-Pugh class B were administered 12 mg of lenvatinib [26]. Patients were administered lenvatinib for a median period of 6.7 months; patients were followed up for 9.1 months (median). In 9% of the patients the disease severity improved, thus requiring a decreased dose. 104 patients in Child-Pugh class A eventually required a smaller dose and decreased overall length of treatment (6.6 months). Additionally, 91 patients in Child-Pugh class B also required a smaller dose and decreased length of treatment (7.3 months) [26]. Towards the final stages of the study, approximately 60.9% of the patients were still taking their tailored dose of lenvatinib. 91 patients discontinued treatment; 39/91 of these patients succumbed to their disease, 32/91 patients started second-line therapy, 20/91 of the patients did not follow up as requested. 32/91 patients who began therapy other than lenvatinib were started on immunotherapy or sorafenib following the termination of lenvatinib [26]. At 6 months and 12 months, progression-free survival rates were assessed and were approximately 85.1% and 64.9%, respectively. Overall survival was also assessed and at 6 months and 12 months were approximately 91.8 and 72.6%, respectively. 70.9% of the patients in this study did not succumb to disease at the time of follow-up. Although this is the first real-world study assessing the effectiveness of lenvatinib as monotherapy in unresectable HCC patients, it is the first step in confirming its effectiveness, thus reassuring clinicians of its therapeutic benefit in clinical settings [26].
Position on lenvatinib management for advanced hepatocellular carcinoma worldwide
Lenvatinib has received worldwide approval as a first-line treatment for patients with HCC since its regulatory approval in 2018 [27]. Since then, 62 countries have authorized its usage with several management guidelines [28]. Fifty-seven countries approved lenvatinib in 2018 and 2019, while Bahrain, New Zealand, Panama, Saudi Arabia, and South Africa approved its usage recently in 2020 [28]. Eisai Global, a research and pharmaceutical company based in Japan, collectively gathered the data for each country that has approved lenvatinib for use in hepatocellular carcinoma and the year of approval, as seen in Figure 2 [28].
Fig. 2
A current world map showing lenvatinib approval for unresectable hepatocellular carcinoma in each country worldwide, as described by Eisai Global. The country, month and year of approval are stated in the legend [29]

The recommended lenvatinib dosages for patients with HCC are dependent on patient weight and are consistent worldwide. Patients weighing > 60 kg are administered a 12 mg dose of lenvatinib orally, once daily, while patients weighing < 60 kg are administered 8 mg orally, once daily [30]. However, the daily doses may be modified by a physician according to the toxicity management plan [30]. Real world analysis of lenvatinib therapy for unresectable HCC shows a similar efficacy and safety profile as patients studied in the REFLECT trial [31]. A retrospective and multiregional study carried out in China by Wang et al. investigated 54 HCC patients on lenvatinib therapy with the majority of patients having a past medical history of hepatitis B [31]. The study showed an ORR of 22% and a PFS of 5.6 months. However, 92.8% of patients presented with adverse effects possibly correlated with the severity of their concomitant hepatitis B related HCC. The study analyzed the characteristics of each patient, serum biomarkers including α-fetoprotein and gene sequencing to gather a complete understanding of lenvatinib therapy. The study concluded with similar findings and safety and efficacy profile as the REFLECT trial with recommendations for larger scale prospective study analysis on patients of varying liver function receiving lenvatinib therapy for unresectable HCC [31].
Limitations
This literature review examined the available information regarding updates to treatment for unresectable HCC using lenvatinib. There is a shortage of long-term research studies on the effects of lenvatinib as a first-line treatment for HCC since its introduction in 2018. The mechanism of action has been elucidated, but the rate of recurrence of the disease has not yet been established. Therefore, this review can only discuss the present knowledge available for the past three years, requiring a later longitudinal study.
Conclusions
Unresectable HCC is limited in its management potential, causing a large number of cirrhosis-related deaths. Stage one HCC and mild cirrhosis have ample treatment options, such as surgical resection, liver transplantation, radio-ablation, transarterial catheter-associated therapy, and systemic therapy, which often provide a better prognosis than late-stage HCC. A phase III clinical trial, REFLECT, indicated the beneficial effects of lenvatinib as first-line therapy for advanced, unresectable HCC, comparing it to the previous first-line therapeutic agent sorafenib, as well as showing a significant response to lenvatinib. With minimal drug-drug interactions and higher response rates to lenvatinib, several countries worldwide have been adopting lenvatinib as a first-line therapy for unresectable HCC since its first approval in 2018. Lenvatinib has been previously accepted for various cancers, such as differentiated thyroid carcinoma, RCC and advanced endometrial carcinoma, and is currently being studied for its use in squamous cell carcinoma of the neck. Thus, with the promising response rate of lenvatinib therapy, additional clinical research is required to fully understand its role and efficacy in treating unresectable hepatocellular carcinoma and potentially generating a complete worldwide approval.






