Introduction
Obesity is a known factor associated with death [1] and is a well-known carcinogenic factor for colorectal cancer [2–4]. Obesity can cause production of vascular endothelial growth factor (VEGF), angiopoietin-2, and other growth factors that induce tumor growth [5, 6]. Cancer progression and carcinogenesis can be modified by chronic inflammation, insulin, leptin, endogenous sex steroids, plasminogen activator inhibitor-1, and adiponectin [7, 8].
However, there is evidence that there is no relationship between obesity and the risk of death in some patients. In addition, there are studies indicating a lower risk of death in patients with certain cancers [9]. This phenomenon is called “obesity paradox”. The beneficial effects of overweight and obesity in some group of patients are suggested [10–12].
BMI is an index assessing body weight and informing about normal or abnormal body weight (underweight, overweight, obesity). Although new tools for assessing overweight and obesity are being studied, for example visceral fat area (VFA) and subcutaneous fat area (SFA), measured with computed tomography, waist circumference [9]. BMI is still a basic and simpler tool for determining normal and abnormal body weight. For this reason, BMI is the most common parameter used in analyses related to the assessment of the effect of body weight on the treatment [9]. Whether body mass index (BMI) is a prognostic or predictive factor in mCRC is unclear. Also, the relationship between obesity and anti-angiogenic treatment has not yet been clearly defined.
Bevacizumab is a humanized monoclonal antibody (mAb) against VEGF and in combination with chemotherapy/immunotherapy is approved for the treatment of advanced colorectal, renal cancer and non-small cell lung cancer, as well as metastatic breast cancer [13]. Its mechanism of action is to selectively bind circulating VEGF and thus to inhibit the interaction of VEGF with its cells receptors. By lowering interstitial pressure in tissues and increasing vascular permeability, the delivery of chemotherapeutic agents into tumor is increase and it leads to tumor endothelial cells apoptosis. On the other hand bevacizumab also causes a reduction in microvascular growth of tumor blood vessels and angiogenesis [13].
Previous studies showed that BMI was associated with prognosis in early-stage CRC, specially low BMI was associated with worse prognosis in metastatic CRC (mCRC) [4]. Other studies have shown that overweight and obesity may be a negative prognostic factor in patients with mCRC and bevacizumab may be less effective in obese mCRC patients [14]. VEGF is a key angiogenic factor that stimulates growth of tumours [15]. Obese people have higher levels of circulating VEGF and for this reason, anti-VEGF therapy can be less effective. There is no consensus about the impact of obesity in bevacizumab-treated mCRC patients. We need more research to understand the relationship between bevacizumab and obesity.
Therefore, the aim of our study was assessment of the associations between BMI and clinical outcomes in patients administered bevacizumab-based treatments for metastatic CRC in the second line treatment.
Material and methods
In this retrospective study, 237 patients with metastatic colorectal cancer treated from January 2014 to August 2018 in 4 reference oncological centers in Poland were included in the analysis. All patients had histopathologically confirmed colon cancer and received bevacizumab with FOLFOX chemotherapy (oxaliplatin, 5-Fluorouracil, leucovorin) as second-line treatment. In the first-line treatment, all patients received irinotecan based chemotherapy (XELIRI or FOLFIRI). Patients who progressed after second line systemic therapy received panitumumab or cetuximab in third line only when mutation in KRAS gene was not present. Presented regimens were standard treatment in Poland in those years.
Complete data were collected on patients such as age, gender, type and location of primary tumor, K-RAS mutation, type of systemic treatment due to mCRC and detailed data on patients at the time of qualification for systemic treatment of the second line, such as fitness status, weight and height, location of metastases, serum CEA (carcinoembryonic antigen) level. All patients had a performance status of 0 or 1 according to ECOG (Eastern Cooperative Oncology Group) [16, 17]. In all patients, imaging was performed every 12 weeks with bevacizumab and FOLOFOX chemotherapy to assess effectiveness of the treatment. Assessments was made using RECIST (Response Criteria in Solid Tumors) version 1.1 [18].
In all patients the BMI index was calculated before the start of bevacizumab treatment with FOLFOX chemotherapy. The BMI index is numerous as the weight in kilograms by the height in meters squared (weight/height2). BMI categories were determined according to WHO criteria (BMI classification World Health Organisation. http://apps.who.int/bmi). Categories BMI: obese BMI ≥ 30 kg/m2, overweight BMI ≥ 25 – <30 kg/m2, normal weight (BMI ≥ 18.5 – < 25 kg/m2 and underweight < 18,5 kg/m2.
Statistical analysis
All statistical analyses were performed using STATISTICA 12. The analysis of variance (ANOVA) was used to determining the significance of impact on quantitative variables, while for qualitative variables chi-squared tests were used to compare probability distributions of patient from different BMI groups. The statistical significance of differences in means for quantitative variables was tested by Student’s t test at the level of significance p = 0.05. The regression analysis (Pearson’s linear correlation coefficient) were used in order to analyzed the relationship between the length of OS and PFS and the BMI value. Test F (Fisher’s exact test) was used to investigate deeper for relationships among variables in multivariate model. Test F allows assessing the importance of the predictors involved in the model. The test value takes into account the sum of squared residuals (error) of the model using a given parameter and the reduced model – without it. In this way, we can determine how much a given predictor improves the predictive quality of the model. The objective of the study was to assess the correlation of BMI value and progression-free survival (PFS) and overall survival (OS) of mCRC treated in second line with bevacizumab with chemotherapy. OS time was calculated from the date of the start of II line treatment to the date of the most recent follow-up or death. PFS time was calculated from the date of the start of II line treatment to the date of the most recent follow-up, or progression or death due to the disease. Radiological response was assessed according to RECIST in third month of treatment The differences were considered statistically significant if the p-values were < 0.05. Survival analyzes including truncated observations were made using Kaplan-Maier models and the Cox hazard model. Both models take into account (in contrast to the previously mentioned methods) the fact that some patients during the analysis are complete observations (fatal), and the remaining records concern living patients whose OS and PFS will be prolonged.
The results of analyzes performed with different methods may differ (and differ) in the aspect of p value due to the different power of statistical tests used in these methods. The juxtaposition of various results was intended to emphasize that, regardless of the method used, the BMI factor is important in predicting the effectiveness of therapy, and the differences may result from the method of discretization (division point and number of groups) or the number of observations in individual classes.
Results
The median age of the patients was 65 years (range 34–82). The cohort included 46% of females and 54% males. The proportion of female patients was higher in the non-obese group (34 vs. 21%, p < 0.001). Other factors like age, location of the primary tumor, the type of treatment in the first line, the level of CEA and the number of locations metastases did not differ depending on the BMI category. Patient characteristics according to BMI are shown in Table 1. Only 3 patients had BMI < 18.5 kg/m2 (18.0–18.4 kg/m2), therefore they were included in the BMI group < 25 kg/m2. The median OS and PFS of the all 237 patients was 14.6 and 8.8 months, respectively. Comparison of obese patient (BMI > 30 kg/m2) vs. overweight patients (BMI ≥ 25 to < 30 kg/m2) vs. normal BMI range patients revealed a significant improvement of median OS (17.5 vs. 14.3 vs. 13.1 months, p = 0.01) (Fig. 1) and median PFS (9.4 vs. 9.1 vs. 7.3 months, p = 0.03) (Fig. 2). There were no differences in the responses to bevacizumab and FOLFOX chemotherapy treatment and number adverse events according to BMI values. Adverse events during treatment were evaluated according to Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 [19]. The most common serious adverse reactions during treatment were neutropenia (G3/G4: 35% patients), polyneuropathy (G2: 10% and G3: 2% patients), diarrhea (G3/G4: 12% patient), proteinuria (G3: 2% patients), hypertension (G3: 5% patients) and thromboembolic events (G3: 5 % patient), intestinal perforation or obstruction (G3: 2% patients). There were no treatment-related deaths. Efficacy and toxicity results FOLFOX + bevacizumab according to BMI are shown in Table 2.
Table 1
Correlation between body mass index level and clinical characteristics of metastatic colorectal patients treated with bevacizumab plus FOLFOX chemotherapy
Table 2
Efficacy and toxicity of bevacizumab plus chemotherapy in metastatic colorectal cancer patients according to BMI in univariate analysis
Fig. 1
Probability of Kaplan-Meier’s progression-free survival (PFS) curves in patients with BMI (body mass index): < 25 kg/m2, 25–30 kg/m2 and > 30 kg/m2 treated with bevacizumab plus chemotherapy
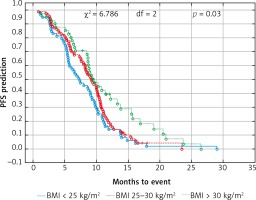
Fig. 2
Probability of Kaplan-Meier’s overall survival (OS) curves in patients with body mass index (BMI): < 25 kg/m2, 25–30 kg/m2 and > 30 kg/m2 treated with bevacizumab plus chemotherapy
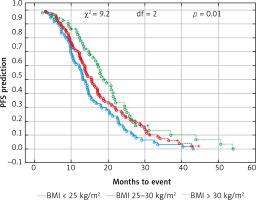
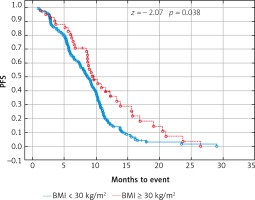
Due to previous studies, two, not three groups of patients with a specific BMI were also compared [14, 20]. Comparison of BMI ≥ 25 kg/m2 group vs. BMI < 25 kg/m2 group and BMI ≥ 30 kg/m2 group vs. BMI < 30 kg/m2 group revealed a significant improvement of median OS (p = 0.017 and p = 0.009 respectively) (Figs. 3 and 4) and median PFS (p = 0.027 and p = 0.038 respectively) (Figs. 5 and 6). Results of treatment in specific groups are presented in Table 3.
Table 3
Median progression-free survival and overall survival according to body mass index categories
| BMI category | PFS (months) | OS (months) | ||
|---|---|---|---|---|
| Median | p-value | Median | p-value | |
| < 25 kg/m2 | 7.3 | 0.075 | 13.1 | 0.061 |
| ≥ 25 kg/m2 | 9.1 | 15.6 | ||
| < 30 kg/m2 | 8.6 | 0.021 | 13.9 | 0.039 |
| ≥ 30 kg/m2 | 9.4 | 17.5 | ||
| Total | 8.8 | 14.6 | ||
Fig. 3
Probability of Kaplan-Meier’s overall survival (OS) curves in patients with body mass index (BMI): < 25 kg/m2 and ≥ 25 kg/m2 treated with bevacizumab plus chemotherapy
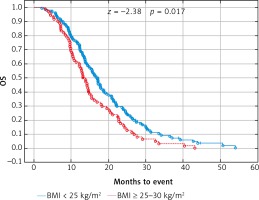
Fig. 4
Probability of Kaplan-Meier’s overall survival (OS) curves in patients with body mass index (BMI): < 30 kg/m2 and ≥ 30 kg/m2 treated with bevacizumab plus chemotherapy
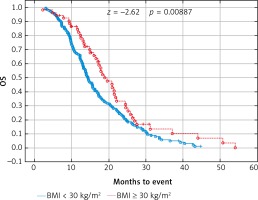
Fig. 5
Probability of Kaplan-Meier’s progression free survival (PFS) curves in patients with body mass index (BMI): < 25 kg/m2 and ≥ 25 kg/m2 treated with bevacizumab plus chemotherapy
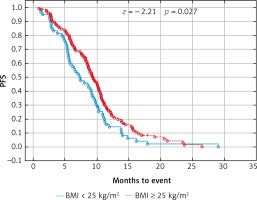
Fig. 6
Probability of Kaplan-Meier’s progression free survival (PFS) curves in patients with body mass index (BMI): < 30 kg/m2 and ≥ 30 kg/m2 treated with bevacizumab plus chemotherapy
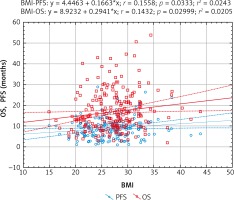
The regression analysis (Pearson’s linear correlation coefficient) also confirmed that there is a statistically significant relationship between the length of OS and PFS and the BMI value. Higher BMI was associated with a better prognosis (Fig. 7).
Fig. 7
Models of overall survival (OS) and progression-free survival (PFS) regression in metastatic colorectal cancer patients treated with bevacizumab plus chemotherapy depending on body mass index (BMI). Study of the significance of the correlation coefficient
The Cox hazard model show that the BMI class is an important risk factor. However, the Cox model also shows that the significance of the BMI class applies only to patients with BMI < 25 kg/m2. This rule applies to both OS and PFS. The Cox model is shown in Table 4.
Table 4
Results of the multivariate Cox proportional hazards models
Multivariate analysis confirmed the statistically significant prolongation of OS and PFS in the group with a BMI ≥ 30.0 kg/m2 vs. BMI < 30.0 kg/m2 and significant prolongation of PFS for patients in the group with a BMI of ≥ 25 – < 30 kg/m2 and a BMI ≥ 30.0 kg/m2 (p = 0.042) but not confirmed the improved OS (p = 0.057). Multivariate analysis not confirmed statistically significant prolongation of OS and PFS in the group with a BMI ≥ 25.0 kg/m2 vs. BMI < 25.0 kg/m2 (p = 0.061 and 0,075 respectively).
Multivariate Cox proportional hazards models and multivariate analysis indicate that objective response (CR; complete response + PR; partial response) to bevacizumab with FOLFOX chemotherapy was associated with a statistically significant increase in OS and PFS. KRAS mutation was associated with worse prognosis in OS and PFS in Cox analysis and only in OS in multivariate analysis. The location of the primary lesion on the left side statistically prolonged OS in Cox and in multivariate analysis. Less than two metastatic sites and CEA within the normal range before the start of bevacizumab with FOLFOX chemotherapy statistically prolonged OS only in multivariate analysis (Table 5).
Table 5
Multivariate analysis (analysis of the significance of predictors) in metastatic colorectal cancer patients treated with bevacizumab plus chemotherapy
Due to the fact that all patients had performance status ECOG 0 or 1 (requirements of the national insurer), no comparisons were made for this factor.
Discussion
BMI is an index assessing body weight and informing about normal or abnormal body weight. Although new tools for assessing overweight and obesity are being studied: VFA, SFA, waist circumference.[9] BMI is still a basic and simpler tool for determining normal and abnormal body weight, for this reason BMI was used in our analysis.
The role of BMI as a predictor and prognostic factor in mCRC is unclear and studied [9, 21]. The prognostic effect of BMI is beginning to be well understood in the early stages of colon cancer, for which both underweight and obese patients have increased risk for progression or death [22, 23]. However, to date, the prognostic and predictive relationships between patients BMI and patient outcomes have been poorly understood in the metastatic setting [4]. It may affect the efficacy of bevacizumab in the treatment of obese patients with mCRC. There are reports of lower efficacy of bevacizumab in obese patients [14, 20] and better effect of bevacizumab in patients with higher BMI [4, 24, 25].
In our study PFS was 8.8 months and the results in a similar study – the Eastern Cooperative Oncology Group Study E3200 study PFS was 7.3 months [26]. It should be concluded that our analysis concerned a very homogeneous group of patients. All patients received bevacizumab with FOLFOX chemotherapy in the second line of mCRC treatment, and as first line treatment they received irinotecan based chemotherapy (XELIRI or FOLFIRI). In contrast, some studies the effect of BMI on the efficacy of bevacizumab related to the treatment of patients in the first or second line due to mCRC [23], or the use of bevacizumab chemotherapy with oxaliplatin, irinotecan or other drug [4, 14, 20, 24, 25, 27].
In the analysis of Artaç [14], patients are divided into two groups with ≥ 30.0 kg/m2 and < 30 kg/mg2 (i.e. obese and non-obese). The analysis of Artaç concerning treatment of mCRC patients with bevacizumab in the first line indicates lower efficacy of bevacizumab in the PFS range in obese patients, however, it does not show such a correlation for OS [14]. In this study, the prognosis was worse in patients with higher body weight only in specific subgroups; left-sided tumor location and the wild type K-RAS. Additionally, in this study, patients received different types of chemotherapy with bevacizumab, which could affect the results. We also do not know what treatment was used after disease progression and whether patients received anti-EGFR [14]. In the analysis of Aykan [20] patients are divided into two groups with BMI ≥ 25 kg/m2 and < 25 kg/mg2 (with increased and normal body weight). Similarly, in this analysis concerning 80 mCRC patients treated with bevacizumab in the first line, patients with BMI < 25 had better PFS than patients with a weight ≥ 25 kg/m2. This study also used bevacizumab in the first or second line of treatment and different chemotherapy regimens which could affect the final results. In connection with analysis of Artaç and Aykan, we performed similar calculations in our group of patients and one of them confirmed the OS and PFS prolongation in the group of patients with BMI < 25 kg/m2 or < 30 kg/m2 analysis. It should be noted that the analyzed our group was very homogeneous, and all patients received the same kind of treatment, and imaging studies performed at 12 weeks. However, the division into two groups of patients seemed insufficient. In our analysis, three groups were identified, including patients with normal body mass < 25 kg/m2, overweight 25–30 and obese > 30 kg/m2. Due to the small number of patients (n = 3) with BMI <18.5 kg/m2, no separate group was assigned for patients with reduced body weight. This division seems more precise and better reflects the impact of weight on bevacizumab treatment. A similar classification was used in a pooled analysis of FFCD trials [24] which included data from 2085 patients enrolled in eight FFCD first-line mCRC trials from 1991 to 2013 was performed [24]. In this trial comparisons were made according to the BMI cut-off: obese (BMI > 30), overweight patients (BMI > 25 – < 30), normal BMI patients (BMI: 18.5–24) and thin patients (BMI < 18.5) [24]. This study confirmed that low BMI is a strong negative prognostic factor. No differences were found between obesity and treatment with bevacizumab, which were indicated by other studies. Similar conclusions were from the ARCAD analysis. This study collected data for 18,564 patients from 21 mCRC first-line studies in 1997-2009. In the ARCAD analysis it was also shown that higher BMI statistically significantly improves PFS and OS in patients with mCRC [25]. In Zafar analysis [27], it was shown that patients with the lowest BMI had shorter median OS, suggesting that low BMI could be a poor-prognostic factor. Simkens in his analysis [28] from the CAIRO and CAIRO 2 study found that BMI is an independent prognostic factor for OS in mCRC receiving chemotherapy. However, this was not confirmed in patients receiving chemotherapy in combination with targeted therapy. In this study, patients received chemotherapy with bevacizumab and / or additional cetuximab as a first line treatment. In this group of patients, a non-significant trend was observed towards improvement of OS when the BMI was higher. This was not confirmed by the FFCD and ACARD analysis. FFCD and ACARD analysis did not show a negative impact on the survival of obese patients treated with bevacizumab in first line treatment mCRC [24, 25]. Our analysis confirms these results in patients with mCRC receiving chemotherapy with bevacizumab as second-line treatment.
It should be noted that in our analyzes, the mutation in the KRAS gene was significantly associated with the overall survival of patients, but the data on the presence or absence of KRAS mutations were not available for 41% of patients. KRAS mutation is an unclear prognostic factor [29] and the effect of KRAS mutations on the survival of patients with mCRC treated with chemotherapy has not been confirmed in other studies [30–32]. However, there are studies indicating that KRAS wild-type may be associated with better prognosis in patients with mCRC receiving capecitabine-based chemotherapy [33].
Our analysis has some limitations due to its retrospective nature, and missing data, but the value of our study is a very homogeneous group of patients and information that higher BMI was associated with statistically significantly better prognosis in patients with mCRC treated with chemotherapy with bevacizumab in second-line treatment was confirmed by several statistical analyzes. It is also one of the very few studies, which shows the relationship of BMI in the second line treatment of chemotherapy with bevacizumab.
Conclusions
Patients with mCRC treated with chemotherapy with bevacizumab in second-line treatment with higher BMI compared with normal weight patients have better prognosis in terms of PFS and OS. In this group, we found no evidence of changes in safety profile depending on BMI. Nevertheless, further large randomized studies are needed to assess the body weight on the effectiveness of chemotherapy in combination with bevacizumab.








