Introduction
The adrenal glands are a major site of metastasis in patients with tumors of the lungs, liver, colon, kidneys, and other organs [1–3]. Non-small-cell lung cancer (NSCLC) is a highly prevalent primary tumor type often characterized by adrenal metastasis (AM), with NSCLC as the primary tumor type in 13–58% of AM patients [1, 2].
AM patients often face poor outcomes, but no clinical trials to date have sought to clarify the benefits of the local treatment of these metastatic lesions [4]. Several reports, however, have indicated that adrenalectomy can improve AM patient overall survival (OS), with a post-surgery median OS of 23–30 months [5, 6]. Together with the design of mini-invasive therapeutic techniques, imaging-guided ablation strategies have successfully been utilized to treat AM [2–4]. Liu et al. [1] additionally determined that imaging-guided ablation and adrenalectomy can achieve comparable OS for AM lesions under 5 cm in size.
Currently, radiofrequency ablation (RFA), cryoablation (CA), and microwave ablation (MWA) have all been established as effective and safe treatment strategies for AM. However, the ablation mechanisms of these methods are different. Whereas MWA and RFA rely on thermal ablation, CA is instead based on the freezing of target lesions. The main advantage of microwave is shorter procedural time and the main advantage of cryoablation is that it is less painful than heat-based ablation [7]. At present, the relative advantages of CA and MWA as treatments for AM remain to be established.
Aim
This study sought to compare the safety and efficacy of CA and MWA as treatments for isolated AM in patients with NSCLC.
Material and methods
This was a retrospective analysis of patients from two centers that received approval from the Ethics Committee of Daxing Hospital and Xuzhou Central Hospital, which waived the need for written informed consent.
Patient inclusion
Between January 2015 and December 2020, consecutive NSCLC patients with AM who underwent CA or MWA treatment were enrolled in this study. To be eligible for inclusion, patients needed to have: (1) a definitive diagnosis of AM secondary to NSCLC; (2) undergone surgical resection for primary NSCLC tumors; (3) AM lesions < 5 cm in size; and (4) refused to undergo adrenalectomy. Patients were excluded in the following cases: (1) extra-adrenal tumors; (2) conditions which were not suitable for ablation; (3) an Eastern Cooperative Oncology Group performance status (ECOG PS) ≥ 3; (4) refusal of ablation; or (5) incomplete clinical data.
The conditions not suitable for ablation were: (1) AM with adjacent vascular, organ, or tissue invasion; or (2) patients with significant dysfunction of blood coagulation, active infection, and/or active bleeding. Both centers performed both of the MWA and CA treatments.
The collected data included patients’ age, gender, tumor history, diameter of AM, side of AM, complete response rate, treatment-related complications, and follow-up outcomes.
Diagnosis criteria
Isolated AM secondary to NSCLC was diagnosed based on a review of each patient’s history, abdominal computed tomography (CT) results, and biopsy findings. The tumor size and side were determined and observed on the CT images.
CT-guided CA
CA was performed under local anesthesia with guidance from a 16-row CT instrument (Philips, OH, USA) using an argon-helium CA device (CryoHit, Galil Medical, Israel). Patients were placed in the prone position, and cryoprobe numbers and distributions were selected in accordance with target tumor position, shape, and size. In cases where the target lesions abutted critical structures, small volumes of ethanol were injected using an 18G needle to displace these structures prior to CA. Electrocardiogram monitoring was maintained throughout the CA procedure.
After cryoprobe placement, tumors underwent two freeze/thaw cycles (freeze for 10 min, thaw for 3 min). Rapid argon gas expansion in sealed cryotubes was used to achieve freezing, achieving a minimum tip temperature of –140°C in seconds. Argon was then displaced by helium to enable thawing. After CA was complete, abdominal CT scanning was repeated to evaluate the patient for evidence of immediate necrosis.
CT-guided MWA
MWA was performed under local anesthesia with a 16-row CT instrument and the KY-2450B MWA system (Kangyou, Nanjing, China). Ablation antennas were placed using the same strategy as was employed for CA treatment, and electrocardiogram monitoring was maintained throughout the MWA procedure.
An ablation power level of 60–70 W and an ablative duration of 4–8 min were selected. Following MWA, abdominal CT scanning was repeated to evaluate the patient for evidence of immediate necrosis.
Follow-up
Patients in this study were hospitalized and monitored for complications, and were discharged within 24 h if they remained complication-free. Long-term follow-up was conducted after 1 week, 1, 3, and 6 months, and every 6 months thereafter. Follow-up analyses included chest and abdominal CT scans, full-body examinations, and brain MRI scans. In addition, bone emission CT was performed every 6 months after ablation. The follow-up was conducted until patients’ death or the point of setting this study (June 2023).
Definitions
Treatment effectiveness was assessed by contrast-enhanced CT and ablation was considered complete if no tumor enhancement was detectable on contrast-enhanced CT scans at 1 week after ablation [8]. If enhancement was still evident, ablation was considered partial. Local progression was defined by evidence of new tumor enhancement in the ablated region [8]. Progression-free survival (PFS) was the interval between ablation and local or extra-adrenal recurrence or progression. OS was the interval from ablation to death or the last follow-up. Hypertensive crisis was defined by a systolic pressure ≥ 180 mm Hg or a diastolic pressure ≥ 110 mm Hg [9, 10].
Statistical analysis
Normally (non-normally) distributed data were presented as means ± standard deviations (medians), and were compared with Student’s t-test (Mann-Whitney U test). Categorical data were compared with the χ2 test and presented in the form of percentages. Median OS and PFS (mOS and mPFS) were compared with the log-rank test. Survival curves were generated by the Kaplan-Meier method. If a patient was lost to follow-up, the PFS and OS were calculated from ablation to the date of the last visit. Predictors of OS were identified via a Cox regression analysis approach. A multivariate analysis was then performed incorporating those variables significantly predictive of OS in univariate analyses (p < 0.1). Potential sources of bias were addressed via subgroup analyses. P < 0.05 served as the cut-off to define significance. SPSS 16.0 (SPSS Inc., IL, USA) was utilized for all statistical testing.
Results
Patients
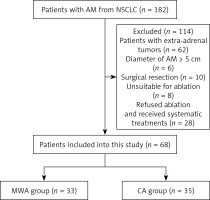
From January 2015 to December 2020, there were 182 NSCLC patients with AM in our centers. This study enrolled 68 patients (Figure 1), of whom 35 and 33 underwent treatment with CA and MWA, respectively (Table I). Baseline patient and tumor data did not differ between these two ablation groups, and each patient exhibited a single isolated AM lesion.
Table I
Baseline data of the patients
Treatment efficacy
Primary complete ablation rates in the CA and MWA groups were comparable at 91.4% (32/35) and 93.9% (31/33), respectively (p = 1.000) (Table II). In patients who experienced partial primary ablation, secondary complete ablation rates were 100% in both groups.
Table II
Treatment outcomes
Complication rates
Hypertensive crisis incidence rates in the CA and MWA groups were comparable at 11.4% (4/35) and 9.1% (3/33) (p = 1.000). In these cases, procedures were suspended and β-blocker, α-blocker, hydralazine hydrochloride, and sodium nitroprusside treatments were administered until blood pressure levels had returned to the normal range, at which time ablation was resumed. Local hemorrhage occurred only in a single patient in the CA group, and this case was managed through hemostasis and anti-inflammatory treatments.
Long-term outcomes
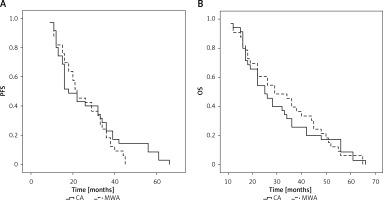
No patients were lost to follow-up after ablation and the median follow-up period was 27 months (11–66 months) for all patients (Table II). Overall, 31 patients (CA group: 18; MWA group: 13) underwent chemotherapeutic treatment following ablation. In total, 8 (22.9%) and 8 (24.2%) patients in the CA and MWA groups experienced local progression over the course of follow-up (p = 0.893), and repeat ablation was performed in all cases. Extra-adrenal metastases were detected in 5 (14.3%) and 5 (15.2%) patients in these respective groups (p = 1.000). The mPFS of patients in the CA and MWA groups was 18 and 22 months, respectively (p = 0.411, Figure 2 A). The 1-, 3-, and 5-year PFS rates of patients in the CA group were 80%, 22.9%, and 8.6%, respectively, while the corresponding rates in the MWA group were 87.9%, 18.2%, and 0.0%.
All patients died over the course of follow-up, with an mOS of 25 and 29 months in the CA and MWA groups, respectively (p = 0.786, Figure 2 B). The 1-, 3-, and 5-year OS rates of patients in the CA group were 94.3%, 25.7%, and 8.6%, respectively, while the corresponding rates in the MWA group were 90.9%, 39.4%, and 6.1%.
Predictors of OS
Univariate Cox regression analyses indicated that lung adenocarcinoma (p < 0.001), previous stage III NSCLC (p = 0.021), and the absence of postoperative chemotherapy (p = 0.042) were all related to shorter OS (Table III). In a multivariate analysis, only a lung adenocarcinoma diagnosis (p < 0.001) was an independent predictor of shorter OS.
Table III
Cox regression analysis for OS
Subgroup analyses
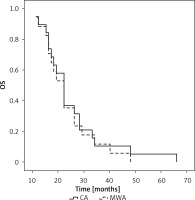
A subgroup analysis was next conducted comparing OS outcomes for individuals with different NSCLC subtypes. The mOS of squamous cell carcinoma patients in the CA and MWA groups was 36 and 45 months, respectively (p = 0.440, Figure 3). The 1-, 3-, and 5-year OS rates of patients in the CA group were 100%, 56.2%, and 12.5%, respectively, while those of patients in the MWA group were 93.8%, 68.8%, and 12.5%, respectively.
Figure 3
Median OS compared between CA and MWA groups for patients with primary lung squamous cell carcinoma
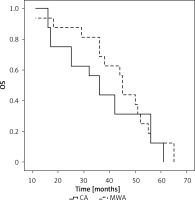
Among adenocarcinoma patients, the mOS in the CA and MWA groups was 22 and 22 months, respectively (p = 0.667, Figure 4). The 1-, 3-, and 5-year OS rates of patients in the CA group were 89.5%, 10.5%, and 5.3%, respectively, while those of patients in the MWA group were 88.2%, 11.8%, and 0.0%, respectively.
Discussion
Effectively treating AM can be difficult and necessitates the selection of an appropriate interventional approach. While surgical resection is the optimal treatment for these metastatic lesions, the efficacy of percutaneous ablation has been reported to be similar to that of surgical resection when AM lesions are smaller than 5 cm [1]. Zhang et al. [2] previously noted that extra-adrenal tumors were predictive of a significant reduction in OS. Patients exhibiting isolated AM are considered the most appropriate candidates for percutaneous ablation.
In this study, the safety and efficacy of CA and MWA were compared as treatments for isolated AM secondary to NSCLC. Comparable primary (91.4% vs. 93.9%, p = 1.000) and secondary (100% vs. 100%) complete ablation rates were observed for these two techniques, suggesting that both ablation strategies are similarly effective as a means of eliminating AM lesions in patients with NSCLC. CA entails the use of alternating freeze/thaw cycles to promote cell death using a range of techniques [11, 12]. Relative to MWA and RFA, CA offers an important advantage of causing lower levels of periprocedural pain [12]. The necessary freeze/thaw cycles, however, prolong the CA treatment time as compared to that associated with RFA or MWA. MWA, in contrast, is a thermal ablation strategy that can more quickly disrupt target lesions.
Hypertensive crisis is a unique complication associated with adrenal tumor treatment [13]. Here, similar rates of hypertensive crisis incidence were observed in the CA and MWA groups (11.4% vs. 9.1%), suggesting that the safety profiles of these two ablation strategies are similar when treating NSCLC patients with isolated AM. The rates in this study are also within the range of the rates which were reported in the prior meta-analyses focused on percutaneous ablation of AM (6–21.2%) [6–8].
Similar rates of local progression (22.9% vs. 24.2%, p = 0.893) and PFS (18 vs. 22 months, p = 0.411) were noted in the CA and MWA groups in this study, suggesting that these ablation techniques can offer similar levels of long-term local control for AM secondary to NSCLC. These rates are comparable to those observed in prior analyses of NSCLC patients with AM undergoing CA (20.5%) and MWA (22.6%) treatment [4, 12]. Botsa et al. [14] similarly observed comparable rates of local control for RFA and MWA (77.2% vs. 80.6%) when used to treat AM secondary to NSCLC. Cheng et al. [4] also found that different subtypes of NSCLC were not associated with local progression (squamous cell carcinoma: 31.3%; adenocarcinoma: 16.6%, p = 0.551).
The mOS of patients in the CA and MWA groups was similar (25 vs. 29 months, p = 0.786), suggesting that the selected ablation technique has no impact on patient OS in cases of AM secondary to NSCLC, as supported by the results of Cox regression analyses. Liu et al. [1] also observed that the mean OS of NSCLC patients with isolated AM following RFA was 2.3 years, which is similar to the mOS in this study. No relationship between postoperative chemotherapy and OS was detected. This may be because all enrolled patients exhibited isolated AM that was effectively controlled through local ablation, and because many of these patients had already undergone chemotherapeutic treatment prior to AM onset such that their tumors may have exhibited higher levels of chemoresistance.
Cox regression analyses revealed that a primary squamous cell carcinoma diagnosis was predictive of longer OS. This may be a consequence of the fact that lymph node metastasis is more common in lung adenocarcinoma cases as compared to squamous cell carcinoma [15]. Kawase et al. [16] further found that cancer-specific death rates associated with lung adenocarcinoma were higher than those for squamous cell carcinoma. When patients were stratified according to NSCLC subtype, however, the mOS of individuals in the CA and MWA groups remained comparable within cohorts of individuals with both squamous cell carcinoma and adenocarcinoma. This suggests that CA and MWA exhibit similar levels of efficacy when treating different NSCLC subtypes.
There are some limitations to this study. For one, the retrospective design entails a high risk of bias and underscores the need for subsequent validation through randomized controlled trials. Moreover, while these patients were derived from two centers, the operative experience levels of personnel at these institutions may have differed, introducing another possible source of bias. Lastly, the sample size was relatively small. However, the specific focus on NSCLC patients with AM may have partially reduced the risk of bias.