Introduction
Left ventricular pseudoaneurysm (LVPA) is a rare but serious complication of myocardial infarction, cardiac surgery, trauma, and infective endocarditis [1]. Although patients can be asymptomatic, LVPA tends to rupture, leading to cardiac tamponade, shock, and death, and therefore requires an urgent repair [2]. In addition to rupture, LVPAs may have risks of thrombosis and coronary artery compression, and thus closure is recommended [3]. If left untreated, LVPA has a high risk of rupture – up to 45% within the first 3 months of LVPA formation [4]. The treatment of LVPA is challenging. Open surgery is the first-line treatment of choice [5]. However, Sakai et al. [6] reported that 7 patients with LVPA formation after mitral valve replacement were not associated with complication during conservative treatment. Percutaneous management of LVPA has been increasingly used in recent decades [7]. Hitherto there has been no systematic report of percutaneous management of LVPA.
Aim
The present article aims to describe the indications, treatment effects, and patient outcomes of percutaneous management of LVPA.
Material and methods
English-language literature was comprehensively retrieved in the PubMed, Google Scholar, and “Baidu” Scholar databases since 2004 [1–81]. The keywords entered in this search to identify articles were “pseudoaneurysm”, “left ventricle”, “left ventricular outflow tract”, “percutaneous”, and “transcatheter”. The inclusion criteria were clinical research, case series, case report, or proceeding abstracts on percutaneous treatment of LVPA of any aetiology. The exclusion criteria were articles describing the following: non-percutaneous treatment of LVPA (n = 21), pseudoaneurysm of other structures (n = 6), percutaneous closure of other defects (n = 4), and percutaneous treatment of LVPA but lack of patient information (n = 1). In total, 32 articles were excluded, and 66 articles were retained.
The data independently extracted from each study were article types, patient demographics, percutaneous manoeuvres and devices, therapeutic effects, and patient outcomes. Extraction of patient information was conducted carefully from each report. This process was replicated 4 times to avoid omissions and ensure the completeness and reliability of the information.
Statistical analysis
IBM SPSS statistics version 22.0 software was used for the statistical analysis. The quantitative data were expressed as mean ± standard deviation and were compared with independent samples t-test. Categorical variables were compared by χ2 or Fisher exact test with continuity correction. P < 0.05 was considered statistically significant.
Results
The 66 recruited articles included 1 (1.5%) retrospective study [22], 4 (6.1%) case series [36, 48, 52, 65], 43 (65.2%) case reports [5, 7, 9–11, 13, 14, 17–21, 24, 25, 27–32, 34, 35, 38–40, 42, 43, 45–47, 49–51, 54, 55, 58, 60, 62, 63, 66–68, 71], 14 (21.2%) medical images [12, 15, 16, 23, 26, 33, 41, 44, 53, 56, 59, 61, 69, 70], and 4 (6.1%) proceeding abstracts/posters [8, 37, 57, 64]. There were a total of 93 patients, including 58 (63.0%) male and 34 (37.0%) female (χ2 = 12.5, p = 0.001), with a male-to-female ratio of 1.7 : 1 (gender of 1 patient was unknown). Six (6.5%) patients were paediatrics [14, 24, 27, 35, 63, 66] and 89 (93.5%) were adults. Their mean age was 63.3 ±20.9 (range: 0.25–90; median: 68.5) years (n = 78). No age difference was noted between male and female patients (60.1 ±24.2 years vs. 65.5 ±18.1 years, p = 0.318).
All patients were diagnosed as LVPA. In 4 (4.3%) patients the LVPAs for percutaneous closure were recurrent [13, 29, 31, 63], and in the remaining patients the LVPAs were primary.
In 1 patient, the mechanism of LVPA formation was not described [64]. Four (4/92, 4.3%) patients had 2 mechanisms for LVPA formation [52, 56, 65], while the LVPA formation in 88 (88/92, 95.7%) patients could be contributed to one mechanism. In short, there were in total 96 mechanisms for 92 patients (Table I). The mechanisms can be divided into surgical, percutaneous, and medial disease related. Of the surgical mechanisms, coronary artery bypass grafting prevailed. As for those owing to a valve operation, there were 12 (54.5%) mitral valve replacements, 7 (31.8%) aortic valve replacements, and 3 (13.6%) double valve replacements (χ2 = 7.4, p = 0.025).
Table I
Mechanisms of LVPA formation
| Mechanism | N (%) |
|---|---|
| Surgical operation: | 104 (76.5) |
| CABG ± left ventricular aneurysmectomy/ventricular septal rupture repair [8, 9, 17, 19, 45, 65, 69] | 47 (45.2) |
| Valve operation ± CABG/aorta operation [5, 11, 14, 22, 23, 26, 30, 36, 53, 54, 62, 67] (for infective endocarditis in 4 patients [14, 26, 30, 36]) | 17 (16.3) |
| Redo valve operation ± CABG [18, 33, 36, 49, 56] | 5 (4.8) |
| Apical left ventricular venting/wire perforation in heart operation [12, 20, 21, 48, 50] | 6 (5.8) |
| Aorta operation [36, 38, 52] | 4 (3.8) |
| Redo aorta operation [28, 52] (for infective endocarditis in 1 patient [52]) | 3 (2.9) |
| Free wall rupture repair [13, 29, 68] | 3 (2.9) |
| Ventricular septal rupture repair [10, 46] | 2 (1.9) |
| LVPA resection [31, 63] | 2 (1.9) |
| Congenital heart defect repair (Ross procedure [24], ventricular septal defect (Swiss-cheese) repair [66] and relief of left ventricular outflow tract obstruction with Brom’s technique [35]) | 3 (2.9) |
| Surgical repair of type II endoleaks [47] | 1 (1.0) |
| Postsurgical, unspecified [37] | 11 (10.6) |
| Percutaneous procedure: | 15 (11.0) |
| Transcatheter aortic valve implant: | 11 (73.3) |
| Transapical [15, 16, 25, 32, 41, 44, 51, 57] | 8 (72.7) |
| Unspecified [7, 34] | 2 (18.2) |
| Via right subclavian access [43] | 1 (9.1) |
| Transcatheter mitral valve implant: | 2 (13.3) |
| Transseptal [59] | 1 (50) |
| Transapical [58] | 1 (50) |
| Balloon aortic valvuloplasty [48] | 1 (0.7) |
| Transcatheter closure of ventricular septal defect and patent ductus arteriosus [27] | 1 (0.7) |
| Medical disease: | 17 (12.5) |
| Myocardial infarction [22, 37, 39, 40, 42, 60, 61, 65, 70, 71] | 12 (70.6) |
| Behcet disease [52, 55] | 3 (17.6) |
| Infective endocarditis [37, 56] | 2 (11.8) |
The formation time of LVPA was reported for 53 patients. In 1 patient, it was described as “a few weeks” [24]. The mean formation time of the remaining 52 patients was 25.6 ±56.5 (range: 0.07–300; median: 4.5) months. The formation time was the longest in medical disease-related, longer in surgical, and shortest in interventional mechanisms of LVPAs, but it did not reach a significant difference (44.4 ±101.7 vs. 29.7 ±56.7 vs. 6.4 ±8.1 months, p = NS). No difference was found between the formation time among the surgical mechanisms, but a decreasing trend from apical left ventricular venting, to aortic, valvular and coronary operations was found (1.8 ±2.0 vs. 22.4 ±14.5 vs. 41.3 ±80.9 vs. 49.2 ±39.0 months, p = NS). The formation time did not differ between patients with a primary surgery and those with a redo operation (29.5 ±60.9 vs. 30.9 ±40.8 months, p = 0.960). The formation time of LVPA did not differ between patients with left ventricular venting during open heart surgery and those with transapical transcatheter valve implant (1.8 ±2.0 vs. 3.2 ±3.9 months, p = 0.566). The formation time due to apical trauma (both transapical transcatheter valve implant and left ventricular venting) was much shorter than that due to other surgical/interventional traumas, but it did not reach statistical significance (2.8 ±3.5 vs. 29.5 ±54.9 months, p = 0.117). The formation time of LVPA due to myocardial infarction was much shorter than due to coronary artery grafting (2.9 ±3.0 vs. 49.2 ±39.0 months, p = 0.029).
Clinical presentations of 52 patients were reported. Ten (19.2%) patients were asymptomatic [7, 31, 35, 36, 41, 43, 51, 52], and the remaining 42 (80.8%) patients had 59 symptoms, with chest pain and dyspnoea/shortness of breath being the most common (Table II). The ejection fraction of the patients was 31.6 ±13.9% (range: 16–55%; median: 25%) (n = 7) [15, 36, 42, 45, 53, 55, 68].
Table II
Presenting symptoms
| Symptom | N (%) |
|---|---|
| Chest pain [9, 10, 11, 18, 20, 25, 36, 45, 47–49, 56] | 12 (20.0) |
| Dyspnoea/shortness of breath [5, 8, 9, 11, 21, 26, 30, 42, 48, 49, 55, 60] | 12 (20.0) |
| Heart failure [17, 19, 21, 23, 30, 39, 40, 54, 61, 64] | 10 (16.7) |
| Growing [6, 11, 20, 24, 40, 63, 66, 68, 71] | 9 (15.0) |
| Hemiparesis [29, 53] | 2 (3.3) |
| Orthopnoea [39, 71] | 2 (3.3) |
| Abdominal pain [67] | 1 (1.7) |
| Acute pulmonary oedema [62] | 1 (1.7) |
| Anorexia [71] | 1 (1.7) |
| Altered consciousness [29] | 1 (1.7) |
| Oedema [14] | 1 (1.7) |
| Fatigue [42] | 1 (1.7) |
| Fever [69] | 1 (1.7) |
| Pulsatile chest wall mass [48] | 1 (1.7) |
| Stroke [29] | 1 (1.7) |
| Syncope [53] | 1 (1.7) |
| Tender, pulsatile epigastric mass [13] | 1 (1.7) |
| Weakness [45] | 1 (1.7) |
| Weight loss [69] | 1 (1.7) |
LVPA growing within a short time was observed in 9 patients, accounting for 9.7% of the entire patient cohort. The observational period for LVPA growth was 3.5 ±1.5 (range: 2–6; median 3.5) weeks (n = 5) [5, 20, 24, 63, 66, 71]. The growth speed of LVPA was 15.0 ±2.6 (range: 12–16.5; median: 16.4) mm/week (n = 3) [5, 66, 71].
For diagnostic purposes, 134 diagnostic techniques were described for 67 patients. Computed tomography and transthoracic echocardiography were the most commonly used diagnostic means (Table III). The overall correct diagnostic rate was 99.3% (133/134), and the misdiagnosis rate was 0.7% (1/134). The only misdiagnosis was made by using an X-ray film, and the LVPA was misdiagnosed as a lung mass, thus leading to a computed tomography-guided biopsy of the mass, revealing blood and cardiac tissue [51].
Table III
Diagnostic techniques
| Diagnostic technique | N (%) |
|---|---|
| Transthoracic echocardiography [5, 8, 9, 12–17, 19, 20, 23–29, 35, 36, 38, 39, 41–43, 45, 46, 48, 49, 53, 55, 57–66, 68–70] | 46 (34.3) |
| Computed tomography [5, 7, 9, 12–14, 16, 17, 20, 23, 26, 31, 35, 36, 38, 43, 48, 50, 52, 54, 56–58, 61, 69] | 29 (21.6) |
| Transoesophageal echocardiography [9, 24, 25, 29, 30, 36, 54, 56, 58, 60, 66] | 14 (10.4) |
| Computed tomographic angiography [10, 11, 18, 21, 28, 30, 31, 46–48, 60, 67, 70] | 13 (9.7) |
| Magnetic resonance imaging [9, 25, 29, 36, 49, 55, 63, 65, 66, 70, 71] | 11 (8.2) |
| Left ventricular angiography [10, 25, 35, 36, 63, 68] | 8 (6.0) |
| Angiography [5, 20, 24, 64, 66] | 5 (3.7) |
| Contrast angiography [64, 66] | 2 (1.5) |
| Magnetic resonance imaging [19, 64] | 2 (1.5) |
| Three-dimensional transthoracic echocardiography [42] | 1 (0.7) |
| Contrast fluoroscopic [5] | 1 (0.7) |
| Computed tomography-guided biopsy [51] | 1 (0.7) |
| X-ray film [51] | 1 (0.7) |
In 14 patients there were one or more associated disorders, which totalled 18 (Table IV). Concurrent procedures were performed in 10 (10.8%) patients: left ventricular apical puncture sealed with another occluder in 2 (25%) [18, 62], coronary artery stenting in 2 (25%) [56, 61], inferior vena cava stenting [14], a 2nd 4-mm ASO deployed across the apical puncture [18], true aneurysm closure with a 10 mm Amplatzer Vascular Plug II [38], coronary artery thrombus aspiration and extracorporeal membrane oxygenation [53], transcatheter mitral valve implant with a 23-mm Edwards Sapien 3 [21], and one-stage simultaneous endovascular repair for abdominal aortic aneurysm and LVPA [67] in 1 patient, each.
Table IV
Eighteen associated disorders
| Associated disorder | N (%) |
|---|---|
| Behcet disease [52, 55] | 3 (16.7) |
| True aneurysm [17, 38, 55] | 3 (16.7) |
| Extrinsic compression of coronary artery ± pulmonary veins [56, 62] | 2 (11.1) |
| Infective endocarditis (of valve-in-valve S3 [58] and of ventricular septal defect patch [69]) | 2 (11.1) |
| Superior vena cava syndrome [55] | 1 (5.6) |
| Inferior vena cava syndrome [14] | 1 (5.6) |
| Cerebral infarct [53] | 1 (5.6) |
| Acute myocardial infarction [53] | 1 (5.6) |
| Coronary artery disease [61] | 1 (5.6) |
| Infrarenal abdominal aortic aneurysm [67] | 1 (5.6) |
| Loeys-Dietz syndrome [38] | 1 (5.6) |
| Small bowel obstruction [16] | 1 (5.6) |
The three-dimensional sizes of the LVPAs were 47.7 ±27.4 (range: 7–130; median: 40) mm (n = 61), 38.1 ±20.4 (range: 8.6–90; median: 34.5) mm (n = 50), and 30.7 ±18.4 (range: 10–90; median: 29.5) mm (n = 18). The neck measured 10.0 ±6.8 (range: 2–32; median: 9) mm (n = 59), and the neck-to-sac ratio was 0.22 ±0.13 (range: 0.03–0.57; median: 0.18) (n = 43).
A mural thrombus inside the LVPA was found in 7 (7.5%) patients [13, 14, 25, 29, 31, 38, 46]. Three (42.9%) of these thrombosed LVPAs were recurrent [13, 29, 31]. One (1.1%) LVPA was ruptured [13]. The locations of LVPAs were reported for 69 LVPAs of 67 patients, and apical LVPA was the most common (Table V).
Table V
The locations of 69 LVPAs of 67 patients
| Location | N (%) |
|---|---|
| Apical [15, 16, 20, 21, 27, 32, 34, 38, 44, 48, 50, 51, 57, 58, 65, 66, 69] | 18 (26.5) |
| Left ventricular outflow tract [11, 18, 24, 26, 28, 33, 36, 52, 56, 62, 63] | 14 (20.6) |
| Anterolateral [22, 25, 31, 43, 49, 60] | 6 (8.8) |
| Paravalve [22] | 5 (7.4) |
| Inferolateral [17, 36, 48, 71] | 4 (5.9) |
| Posterolateral [9, 13, 36, 64] | 4 (5.9) |
| Lateral [23, 46, 48] | 3 (4.4) |
| Posterior [14, 35, 42] | 2 (2.9) |
| Posteroapical [19, 67] | 2 (2.9) |
| Posterobasal [30, 36] | 2 (2.9) |
| Anterior [47] | 1 (1.5) |
| Anteroapical [22] | 1 (1.5) |
| Basal inferolateral [39] | 1 (1.5) |
| Inferior [61] | 1 (1.5) |
| Inferior and inferolateral [55] | 1 (1.5) |
| Lateral apical [29] | 1 (1.5) |
| Posteroinferior [45] | 1 (1.5) |
| Posteromedial [5] | 1 (1.5) |
The indications for percutaneous treatment of LVPAs were described for 42 patients and previous operations/sternotomies were a prevailing factor (Table VI).
Table VI
Indications for percutaneous treatment of LVPAs in 42 patients
| Indications | N (%) |
|---|---|
| Previous operations/sternotomies [13, 21, 26, 28, 30, 31, 36, 45, 46, 49, 50, 52, 65] | 13 (25.0) |
| High surgical risk [7, 16, 19, 24, 39, 53, 55, 56, 60, 67, 69] | 11 (21.2) |
| LVPA growing [5, 11, 20, 24, 40, 63, 66, 68, 71] | 9 (17.3) |
| Patient’s comorbidities [9, 23, 47, 65] | 4 (7.7) |
| Impending LVPA rupture [15, 50, 65] | 3 (5.8) |
| High risk of operative mortality [10, 30] | 2 (3.8) |
| Despite optimal medical therapy, symptoms of heart failure remained [17] | 1 (1.9) |
| PA was not enlarged but patient felt chest pain during a 6-month follow-up [25] | 1 (1.9) |
| Expected technical difficulties with surgical repair [20] | 1 (1.9) |
| Patient’s advanced age [36] | 1 (1.9) |
| Preventing further cardioembolic events [29] | 1 (1.9) |
| Reducing the risk of aneurysm rupture [29] | 1 (1.9) |
| Previous myocardial infarction with reduced left ventricle function [46] | 1 (1.9) |
| Pseudoaneurysm’s geometry [47] | 1 (1.9) |
| Recent surgical treatment [48] | 1 (1.9) |
| Thoracotomy for hybrid apical access posing a risk of exsanguination [62]. | 1 (1.9) |
The percutaneous closure of LVPA was performed on an urgent basis in 3 (3.2%) patients [13, 20, 23]. The percutaneous procedures succeeded on the first try in 79 (84.9%) patients, whereas failures were encountered during the percutaneous manoeuvres in 14 (15.1%) patients [10, 11, 14, 24, 30, 44, 51, 54, 63–67, 70]. Of the latter 14 patients, the percutaneous closure of LVPA eventually succeeded in 12 (85.7%) patients, whereas it had to convert to open surgery in 2 (14.3%) patients [66, 67]. In total, 86 percutaneous approaches were described for 77 patients (Table VII). Of them, percutaneous manoeuvre succeeded on the first try in 72 patients in whom percutaneous approaches were attempted once, while in 5 patients the percutaneous procedure failed 1–3 times, with a total of 8 failures and therefore with 8 more approaches. Another patient who had 2 LVPAs required percutaneous therapies twice and thus with one more approach [20]. Of the 8 failures, 5 (62.5%) were retrograde [10, 44, 51,65, 70] and 3 (37.5%) were antegrade approaches [44, 51].
Table VII
Eighty-six percutaneous approaches in 77 patients
| Percutaneous approach | N (%) |
|---|---|
| Antegrade: | 34 (39.5) |
| Transapical [11, 18, 22, 34, 37, 44, 48, 51, 54, 62] | 23 |
| Transseptal [26, 41, 56, 64] | 4 |
| Ante apical puncture through mini-thoracotomy [33, 40] | 2 |
| Right femoral vein [31] | 1 |
| Right jugular vein [10] | 1 |
| Direct chest wall puncture [37, 70] | 3 |
| Retrograde: | 47 (54.7%) |
| Right femoral artery [5, 7, 13, 16, 17, 25, 28, 49–51, 53, 65] | 12 |
| Right common femoral artery [12, 20, 38, 60] | 5 |
| Left femoral artery [10, 30, 43, 68] | 4 |
| Femoral artery [19, 23, 32, 45, 48, 51, 63] | 8 |
| Retrograde aortic approach [37, 44, 70] | 4 |
| Left brachial artery [67] | 1 |
| Right brachial artery [65] | 1 |
| Left carotid artery [71] | 1 |
| Retrograde, unspecified [21, 22, 35, 36, 39, 42, 55, 58, 59] | 11 |
| Antegrade and retrograde: | 5 (5.8) |
| Left femoral vein and right femoral artery [66] | 1 |
| Right femoral artery and right femoral vein [46] | 1 |
| Retrograde/transapical [22] | 3 |
In 1 patient, the device used was not described. The devices that were used for LVPA closures included 65 septal occluders, 18 plugs, and 16 groups of embolisation coils. The Amplatzer muscular ventricular septal defect occluder was the most commonly used (Table VIII).
Table VIII
The devices for closures of left ventricular pseudoaneurysm
| Device | N (%) |
|---|---|
| Septal device: | 65 (64.4) |
| Amplatzer Muscular Ventricular Septal Defect Occluder [7, 13, 15, 16, 23, 28, 29, 31, 32, 41, 43, 44, 47–49, 53, 56, 57, 59, 69, 70] | 22 (33.8) |
| Amplatzer Septal Occluder [18–20, 22, 24, 36, 39, 42, 44, 46, 48, 58, 60, 61, 64, 67, 68] | 15 (23.1) |
| Amplatzer ASD Occluder [8, 10, 27] | 3 (4.6) |
| Muscular Ventricular Septal Defect Occluder (MVSDO, Lifetech Ltd., Shenzhen, China) [65] | 2 (3.1) |
| Amplatzer Duct Occluder [22] | 1 (1.5) |
| Amplatzer Cribriform Occluders [44] | 1 (1.5) |
| Amplatzer Muscular Septal Occluder [55] | 1 (1.5) |
| Amplatzer Duct Occluder II (ADO II) [66] | 1 (1.5) |
| Amplatzer devices, unspecified [37] | 14 (21.5) |
| Atrial septal occlusion device (SHSMA, Shanghai, China) [71] | 1 (1.5) |
| CERATM ASD (Lifetech Scientific Inc., Shenzhen, China) device [40] | 1 (1.5) |
| Duct occluder [62] | 1 (1.5) |
| MemoPart Ventricular Septal Defect® (VSD) occluder (Idoramed) [17] | 1 (1.5) |
| Occlutech Figulla II ASD Occluder [30] | 1 (1.5) |
| Coils [9, 24, 36, 44, 52, 63, 70] | 16 (15.8) |
| Plug: | 18 (17.8) |
| Amplatz Vascular Plug II [9, 11, 12, 21, 22, 25, 33, 34, 35, 38, 45, 51] | 13 (72.2) |
| Amplatzer Vascular Plug III [6] | 1 (5.6) |
| Amplatzer Vascular Plug 4 [14, 26, 50, 66] | 4 (22.2) |
| Edwards Sapien XT for transcatheter aortic valve implant [54] | 1 (1.0) |
| Unknown [37] | 1 (1.0) |
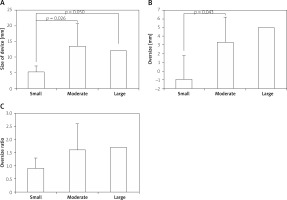
The dimensions of septal devices were divided into 3 groups: moderate (well suited for closure of LVPA neck), small, and large. The size of the devices was much greater in moderate than in small groups (p = 0.026). A difference was found between small and large (p = 0.050). A difference was found in the oversize of the devices between moderate and small groups. The oversize ratio of the small devices was much less than that of the moderate devices, but without reaching a significant difference (Figure 1).
Figure 1
Comparisons of parameters of device choices: A – size, B – oversize; C – oversize ratio between the small-, moderate-, and large-sized devices
Guidance of the percutaneous manoeuvre was described for 44 patients; transoesophageal echocardiography was most commonly used (Table IX).
Table IX
Guidance for percutaneous manoeuvres
| Guidance | N (%) |
|---|---|
| TEE [5, 7, 9, 10, 15, 17, 25, 28, 33, 41, 46, 60] | 12 (27.3) |
| Fluoroscopy and TEE [13, 16, 21, 24, 29, 43, 47, 51, 54, 55] | 10 (22.7) |
| Fluoroscopy [18, 38, 48, 50, 63, 67, 68, 70] | 8 (18.2) |
| echocardiographic and fluoroscopy [11, 19, 65] | 3 (6.8) |
| Fluoroscopic and TTE [61, 58, 45] | 3 (6.8) |
| TTE [30, 35, 39] | 3 (6.8) |
| Fluoroscopy and intracardiac echocardiography [42, 56] | 2 (4.5) |
| Computed tomography–fluoroscopy [44] | 1 (2.3) |
| Fluoroscopy and combined TTE/TEE [23] | 1 (2.3) |
| Selective hand angiography [14] | 1 (2.3) |
There were 2 LVPAs in 3 (3.2%) patients [20, 24, 30]. Therefore, there were in total 96 LVPAs in 93 patients. There were 121 wire accesses, with 10 (10.8%, 10/93) patients having 14 (11.6%, 14/121) failed accesses.
The causes of failures were described for 9 chances of 7 patients: tortuosity of the aorta (n = 2) [31, 65], difficulty with crossing the LVPA neck (n = 3) [30, 51], unable to maintain position (n = 1) [51], adequate length, angle, and loop of delivery catheter for stable device deployment (n = 1) [14], delivery sheaths too small (n = 1) [10], and poor visualisation of the exact localisation of the channel to the LVPA at angiography (n = 1) [20].
In 10 patients, 2 or 3 devices were used for one LVPA. In 5 of them, the closures for a single LVPA required more devices [9, 24, 66, 67, 69]. In 5 patients, the requirement of more devices was due to closure failures [11, 30, 37, 44, 63]. In addition, one of the two LVPAs in a patient was not treated and no device was used [30].
There were in total 106 closures for 95 LVPAs (one LVPA was not managed due to small size). Eight patients had closure failures, with one failure in 5 patients [24, 30, 64, 66, 70] and 2 failures in 3 patients [22, 44, 67] (there was an additional open patch repair failure in 1 patient [44]). In addition, surgical open external suture of an LVPA failed in 1 patient [14]. The causes of closure failures were described for 6 patients: device too small or too big (n = 2) [11, 30], problematic design of the device (n = 2) [64, 66], the trabecula over the neck hindering a complete seal (n = 10) [67], and partial/complete extrusion of device as well as pulling the errant coil during the manoeuvres [63]. The time interval between procedures in the same patients (staged, redo after failure, and procedures for closures of true and false aneurysms) was 69.0 ±74.0 (range: 10–180; median: 30) days (n = 5) [14, 20, 38, 63].
Effects of percutaneous closure for LVPA cavity were reported as follows: complete exclusion of the LVPA (n = 8) [7, 12, 15, 28, 33, 44, 45, 50], complete thrombosis of LVPA with no residual flow (n = 7) [11, 26, 34, 41, 46, 55, 61], contraction of the pseudoaneurysm (n = 1) [69], partial thrombosis of LVPA (n = 1) [7], no blood communication to LVPA by thrombin injection [51, 37], large LVPA sacs filled with embolisation coils (n = 3) [37], LVPV reduced in diameter [6], and LVPA persistent but not increasing in size and partially thrombosed [39].
Minimal residual flow immediate after closure could be observed in 13 patients [5, 20, 29, 39, 43, 48, 49, 54, 57, 60, 65, 68, 71], and on day 2 in 2 patients [66, 67]. No flow was observed 41 patients 16.7 ±37.8 (range: 0–180; median: 0) days after closure [5, 7, 9, 13, 14, 16–22, 24–29, 31, 33, 38, 41–44, 46–49, 51, 55–58, 60, 61, 65, 66, 68, 70].
Patients’ hospital stay after percutaneous LVPA closure was 5.3 ±6.0 (range: 1–28; median: 3) days (n = 26) [5, 7, 8, 11, 12, 16–18, 21, 25, 27, 28, 31, 34, 36, 38, 40, 41, 45, 46, 48, 49, 51, 56, 58, 60]. Patients were on a follow-up of 12.4 ±19.4 (range: 1–84; median: 6) months (n = 52) [5, 7, 9–15, 17, 18, 20–22, 24–29, 31, 34–39, 42, 43, 46–49, 52, 54–62, 65, 66, 68, 69, 71]. Outcomes were known for 71 patients: 41 (57.7%) recovered [8, 11, 12, 14–18, 20, 21, 24, 25, 28, 34–38, 41, 46–49, 51, 53, 54, 57–60, 62, 63, 65, 66, 68–70], 17 (23.9%) improved [22, 23, 37, 39], and 7 (9.9%) were complicated (including coils lost in the false lumen without clinical sequelae and residual leak [36], access site left femoral artery bleeding [30], aphasia, reversible ischaemic neurologic deficit [48], jaundice, haemolytic anaemia, hyperbilirubinaemia, and acute renal failure [67], mechanical compression of circumflex by occluder [36], and post-procedural pericardial effusion [27]). All complications resolved after proper managements. Six (8.5%) patients died: 2 early deaths [29, 64], 2 late deaths [48, 71], and 2 deaths with time not given [37]. The causes of death were recurrent pulmonary emboli [29], bronchial fistula [37], progressive congestive heart failure [37], unrelated causes [48], multiorgan failure [71], and reason not given [64].
Discussion
LVPA may be iatrogenic (previous cardiothoracic surgery, most commonly mitral valve replacement), traumatic (chest trauma), infective (inflammatory/autoimmune disorders), or because of myocardial infarction [72]. Postoperative LVPAs occur in 0.02–2% of mitral valve operations [73]. Other causes of LVPAs include intraoperative venting of the cardiac apex, penetrating trauma, and infection [74]. The pathogenesis of LVPA in Behçet disease is unclear, but there are 2 hypotheses: a subclinical coronary thrombotic event leading to necrosis of the inferolateral myocardium with subsequent development of LVPA; and focal myocarditic processes initially leading to the true aneurysm formation with subsequent sac expansion [55].
Patients with an LVPA can be asymptomatic and are diagnosed incidentally [75]. Severe patients may present with congestive heart failure, arrhythmias, thrombosis, or cardiac rupture [76].
Echocardiography is the most common modality for the diagnosis of LVPAs. Transoesophageal three-dimensional echocardiography improves the diagnostic accuracy. Postinfarction LVPAs are diagnosed during cardiac catheterisation by left ventriculography. Computed tomography and magnetic resonance imaging are helpful in differentiation between false and true aneurysms [77].
Chronic, asymptomatic LVPA < 30 mm in diameter could be treated conservatively [78]. Symptomatic patients and those with larger LVPAs of impending rupture warrant closure [78]. If an LVPA develops within 2–3 months after myocardial infarction, an urgent operation is warranted owing to the high risk of rupture [79]. Surgical repair is the mainstay of treatment of choice. Surgical techniques include LVPA resection neck closure by primary suture or patch repair. Transcatheter closure of the pseudoaneurysm is done especially in patients deemed to be at high surgical risk [77]. The first percutaneous LVPA closure was described by Clift et al. in 2004 with the use of a 12 mm Amplatzer septal occluder [19]. Percutaneous closures were achieved with septal occlusion devices, coils, and vascular plugs depending on the anatomy. Multimodal imaging is critical for determining the precise location and relationship of the pseudoaneurysm to the surrounding structures, and it improves the success rate of the percutaneous manoeuvre [77].
As for the percutaneous approaches, the transapical approach provides a shorter course than the transseptal approach for accessing left ventricular outflow tract. Thus, transapical closure is an efficient and safe option for left ventricular outflow tract pseudoaneurysm [33]. The present study revealed that the antegrade transapical approach was the most common irrespective locations of LVPAs.
Treatment options include Amplatzer devices, vascular plugs (for moderate- to large-sized pseudoaneurysms with narrow necks), and coil embolisation (for small- to moderate-sized LVPAs with narrow necks, and for cases that raise concern about the compressive effects of occluder devices) [54]. Al-Hijji et al. [11] tested different sizes of Amplatz Vascular Plug II devices: the 8-mm device was too small to offer complete seal of the neck, the 12-mm device offered complete seal but it was too large and protruded into the prosthetic aortic valve, whereas the 10-mm device offered a complete seal and no risk of aortic prosthesis obstruction. Moriarty et al. [47] recommended the muscular VSD device oversizing by at least 5 or 6 mm, considering transient neck enlargement and mobility during device placement. The target neck size should be 10–12 mm because the maximal device waist is 18 mm [47]. However, Vascular plugs are usually not recommended for use because they do not have sufficient stability as septal occluders for LVPA closure, and most plugs do not have a sufficient waist [72]. In addition, transapical deployment of an Amplatzer septal occluder with the left atrial disk hung in the left ventricle apex and the right atrial disk filling the neck remaining incompletely expanded [48]. Transapical access had been established and the pseudoaneurysm contributed to regurgitant blood flow, and thus an Edwards Sapien XT transcatheter heart valve in valve-in-valve fashion was implanted for closure of the neck instead of an Amplatzer plug [54]. Our patient was eligible for percutaneous closure, but placing an Amplatzer plug was not technically feasible. Placing an Edwards Sapien XT transcatheter valve enabled thrombosis of the LVPA [54].
Untreated LVPA has 30–45% risk of rupture [4], and with medical therapy the mortality is 48% [51]. Even with surgical intervention there is a high mortality rate of up to 35% [80]. The overall hospital mortality of surgical repair of LVPA was 27.3% and mean survival was 61.9 ±41.4 months in the 16 hospital survivors [79]. Moreno et al. [81] reported 1-year and 4-year survival rates of 88.9% and 74.1% in conservatively treated patients, respectively. In this study, percutaneous closure was associated with a mortality rate of 8.5%.
Incomplete patient data was the main shortcoming of the present study. The information of size and oversize of the failed devices merely offered a rough comparison between successful and failed cases. Thus, the cut-off valves of oversize 3.3 mm and of oversize ratio 1.6 preliminarily obtained were references of suitable device choices. Post-procedural ejection fractions were not reported, and thus the assessment of the effect of percutaneous treatment on the left ventricular function was impossible. Total procedural time and fluoroscopy time were described in very few reports. Thus, the complexity of deployments of different devices could not be further evaluated.
Conclusions
Percutaneous closure of LVPA was especially indicated for patients carrying a high surgical risk. Iatrogenic traumas, such as left ventricular venting, should be avoided in order to eliminate this complication. For device choice, the cut-off valves of oversize 3.3 mm and oversize ratio 1.6 should be kept in mind for reference.








