Introduction
Atrial fibrillation (AF) is the most commonly observed arrhythmia in the clinical practice, affecting 33.5 million patients worldwide [1]. There is a 5-fold higher risk of cerebrovascular thromboembolic events causing disabling symptoms, with higher mortality compared to other stroke etiologies [2].
Oral anticoagulation (OAC) is the first line treatment in stroke prevention in patient with AF, and regarding the current guidelines direct oral anticoagulants (DOACs) are recommended over vitamin K antagonists (VKA) [3]. Despite the promising safety profile of DOACs, the rate of major bleeding observed in randomized clinical trials remains high [4]. Bleeding is the main cause of under-treatment with OAC therapy. Data show that up to 50% of patients with an indication for OAC therapy are left unprotected because of previous bleeding complication or high bleeding risk [5].
The left atrial appendage (LAA) is considered to have the highest thrombotic potential and probably most of the strokes in patients with AF are caused by thrombus that originated from the LAA [6]. In a review of 23 studies, it was found that more than 90% of atrial thrombi are located in LAA in patients with non-valvular AF [7]. Based on this evidence, left atrial appendage closure (LAAC) was offered as an alternative approach to reduce cardioembolic risk in patients with AF [8]. This transcatheter method was developed to exclude LAA from the blood circulation, preventing the release of the thrombus from its cavity. Such a strategy seems to prevent AF-driven ischemic stroke while overcoming the challenges associated with OAC therapy and reducing long-term bleeding risk [9]. Currently, European guidelines recommend LAAC in patients with non-valvular AF with contraindications to long-term OAC therapy (class IIb indication, level of evidence B) [3].
The Watchman 2.5 (Boston Scientific, Natick, MA, USA) is the most frequently used device for LAAC in the world [10]. Long-term safety and efficacy have been proven in three randomized clinical trials and several registries. In 2019, the second-generation Watchman FLX was presented with the intention to ameliorate LAA sealing, decrease the risk of device-related thrombus and to simplify the implantation of the device. The aim of this review is to summarize the current evidence on transcatheter LAAC with the Watchman 2.5 and Watchman FLX devices.
LAA anatomy and thrombus formation
The LAA is a trabecular structure which is a residue of the original embryonic left atrium (Figure 1) [11]. It is composed of an orifice, neck and body. The body of the LAA is trabeculated with pectinate muscles. The cavities protruding out of the body of the LAA are defined as lobes. The size and shape are different in each patient. Its morphology is classified into four types assessed with transesophageal echocardiography (TEE) or computed tomography (CT), which is superior to TEE [12]. The dominant shape is “chicken wing” (48%), then “cactus” (30%), “windsock” (19%), and “cauliflower” (3%) [13]. The latter shape was found to be most often associated with embolic events, while the most common one, “chicken wing”, has the lowest risk for thrombus formation [13]. During AF there is an increased maximal appendage size, and decreased filling and emptying velocities causing blood stasis in its cavity. This state predispose to thrombus formation. Moreover, the morphology of LAA has a significant influence on the risk of stroke. Yamamoto et al. found that larger volume and depth of LAA, number of lobes, and extensive LAA trabeculation were associated with higher risk of stroke and transient ischemic attack (TIA) [14].
Indication for the procedure
LAAC with Watchman device implantation is an alternative method to anticoagulation therapy in order to prevent thromboembolic complications in patients with AF. Both American and European guidelines provide a class IIb recommendation for considering LAAC in patients at high risk of stroke and contraindications to long-term OAC therapy [3, 15]. The indications and contraindications for LAAC are presented in Table I.
Table I
Indications and contraindications for percutaneous LAAC
Watchman 2.5 device
The Watchman 2.5 occluder is the most extensively studied LAAC device in the world and the only one whose safety and efficacy have been assessed in randomized trials (Table II). It is self-expanding, consisted of a 10-strut nitinol frame which is covered on its atrial surface with a polyethylene terephthalate (PET) membrane that ameliorates device endothelialization (Figure 2) [16]. The open distal part contains 10 anchors responsible for the device’s fixation to the LAA. There are 5 available Watchman device sizes to suite the different types of LAA and they range from 21 mm to 33 mm (Figure 2). The device was designed to occlude an LAA with a diameter from around 17 mm to 31 mm; a Watchman 2.5 occluder that is 8% to 20% larger than the LAA ostium is selected to obtain adequate compression. The depth of the LAA should equal the diameter of the device when fully expanded. Moreover, the device can be partially or fully recaptured to change the deployment position. In March 2015, based on the data from two randomized trials, the US Food and Drug Administration (FDA) approved the Watchman for LAAC to reduce the risk of stroke in patients with non-valvular AF.
Table II
Main studies evaluating outcomes of the Watchman device
| Characteristics | PROTECT AF, 200917 | PROTECT AF, 2014 | PREVAIL, 201419 | CAP and CAP2, 201921 | ASAP, 201323 | EWOLUTION, 201725 | EWOLUTION, 2019 | PINACLE FLX, 202030 | FLXibility study, 202332 |
|---|---|---|---|---|---|---|---|---|---|
| Study design | RCT 2 : 1 | RCT 2 : 1 | MCR | MCR | MCR | MCR | MCR | ||
| Device group: control group, n | 463 : 244 | 269 : 138 | |||||||
| Device group, n | 1144 | 150 | 1025 | 400 | 300 | ||||
| CHADS2, mean (SD) | 2.2 (1.2) | 2.6 (1.1) | 2.6 (1.2) | 2.8 (1.2) | NA | NA | NA | ||
| CHA2DS2VASc, mean (SD) | NA | 3.8 (1.2) | 4.2 (1.5) | 4.4 (1.7) | 4.5 (1.6) | 4.2 (1.5) | 4.3 (1.6) | ||
| Follow-up, mean (SD) | 18 (10) | 47 (20) | 12 (6) | 50 | 14 (9) | 12 | 24 | 12 | 12 |
| Post-procedural antithrombotic regimen | VKA+ ASA, 45 days ASA + clopidogrel, 6 months ASA, lifelong | DAPT, 6 months ASA, lifelong | DAPT, 60% SAPT, 7% VKA, 16% DOAC, 11%, none 6% | DOAC + ASA, 45 days DAPT, 6 months ASA, lifelong | DAPT, 87% SAPT, 7% DOAC, 5% VKA, < 1% none, < 1% | ||||
| Implant success, % | 88 | 95.1 | 94 | 94.7 | 98.5 | 98.8 | 99 | ||
| Periprocedural* major adverse event, % | 8.7 | 4.2 | 4.2 | 8.7 | 2.8 | 0.5 | 2 | ||
| Procedure-related stroke, % | 1.1 | 0.4 | 0.2 | 0 | 0 | 0.5 | 0 | ||
| Procedure-related death, % | 0 | 0 | 0.2 | 0 | 0.4 | 0 | 0 | ||
| Pericardial effusion, % | 4.8 | 1.9 | 2.2 | 1.3 | 0.3 | 0 | 1 | ||
| Nonprocedural major bleeding, % | 3.5 | 4.1 | NA | 6.9 | NA | 2.3/year | 2.7/100 patient-years | 7.9 | 6.8 |
| Hemorrhagic stroke, % | 0.1/100 patient-years | 0.6 | 0.4 | 0.13/100 patient-years | 0.6/100 patient-years | 0 | 0.2 | 0 | 0 |
| Ischemic stroke/TIA/SE, % | 2.5/100 patient-years | 1.6/100 patient-years | 2.3 | 1.8/100 patient-years | 2.3/100 patient-years | 1.5/100 patient-years | 2/100 patient-years | 2.9 | 2.7 |
| Mortality, % | 3/100 patient-years | 3.2/100 patient-years | 2.6 | 5.4/100 patient-years | 5/year | 9.8 | 15.7 | 6.6 | 10.8 |
| Device embolization, % | 0.6 | 0.7 | 0.2 | 1.3 | 0.2 | 0 | 0.3 | ||
| Device-related thrombus, % | 4.2 | NA | 3.3 | 4 | 3.7 | 4.1 | 1.8 | 2.4 | |
Figure 2
Comparison of Watchman 2.5 and Watchman FLX and the sizing charts. Graphic based on images received by courtesy of Boston Scientific Corporation
PET – polyethylene terephthalate.

The PROTECT-AF trial was the first, multicenter, randomized clinical trial (RCT) designed to assess the safety and efficacy of the Watchman device [17]. This non-inferiority RCT enrolled 707 patients with non-valvular AF and a CHADS2 score ≥ 1 and randomized them in a two-to-one allocation to receive either device closure of the LAA with Watchman or VKA therapy with warfarin. After successful Watchman implantation all patients received warfarin for 45 days followed by dual antiplatelet therapy (DAPT) with aspirin and clopidogrel for 6 months after the procedure and then lifelong aspirin monotherapy. The primary efficacy endpoint was a composite of stroke, systemic embolism and cardiovascular death. The trial showed that LAAC with the Watchman device is non-inferior to warfarin therapy [17]. This result was mainly driven by a significant reduction in hemorrhagic stroke (Watchman: 0.6% per 100 patient-years vs warfarin: 4% per 100 patient-years) and cardiovascular death (Watchman: 1% per 100 patient-years vs warfarin: 2.6% per 100 patient-years). Ischemic stroke did not reach non-inferiority, but it has been demonstrated that LAAC significantly decreased the rate of fatal and disabling strokes (Watchman: 0.5% per 100 patient-years vs. warfarin: 1.2% per 100 patient-years). The primary safety endpoints (composite of serious bleeding, cardiac tamponade, device embolization, procedure-related stroke) was observed at a higher rate in the intervention group (7.4%) than in the control group (4.4%) (RR = 1.69, 95% CI: 1.01–3.19) with a rate of 4.8% of pericardial effusion that required percutaneous or surgical intervention. None of these patient died; however, patients with pericardial effusion required longer in-hospital stay. The impact of the operator’s experience on the safety of the LAAC procedure was evaluated in the analysis of the intervention arm from the PROTECT-AF trial (n = 542) and from a subsequent registry of patients undergoing Watchman implantation – Continued Access to PROTECT-AF (CAP) (n = 460). There was a significant decrease in the rate of safety events between the first and second half of the PROTECT-AF and CAP patients [18]. Procedure-related safety events within 7 days in the PROTECT-AF trial and CAP registry was 7.7% and 3.7% (p = 0.007), respectively. The same event rate in the first and the second half of PROTECT-AF patients was observed in 10% and 5.5% (p = 0.006), respectively.
The FDA raised a couple of concerns regarding patients’ selection criteria and safety profile of LAAC with the Watchman device. In response to these concerns, the PREVAIL trial was designed specifically to further evaluate the safety of the device and to confirm its efficacy observed in the PROTECT-AF trial [19]. It was a non-inferiority trial of 407 subjects who were randomized in two-to-one fashion either to the Watchman device or warfarin therapy. Similarly to PROTECT-AF, the first primary efficacy endpoint was a composite of stroke, systemic embolism, and cardiovascular death. A second, co-primary endpoint was stroke and systemic embolism occurring > 7 days after the procedure. The safety endpoint was a composite of a pre-defined performance criterion set by the FDA including death, ischemic stroke, systemic embolism, and procedure-device related complications requiring major intervention within 7 days after the procedure. The CHADS2 score was higher than in the first trial with a score of 2.6 ±1.0 in both groups, and as pre-specified, 38.8% of the patients were randomized at new sites and 39.1% of the procedures were done by new operators. At 18 months, the trial failed to show non-inferiority regarding the primary efficacy endpoint. The rate of the second co-primary efficacy endpoint met non-inferiority criteria. Importantly, the PREVAIL trial showed an improved safety profile in comparison to the PROTECT-AF trial, with only a 2.2% rate of procedural complications [19]. Furthermore, the procedure success rate increased from 90.9% in PROTECT-AF to 95.1% in PREVAIL.
In 2017 a patient-level meta-analysis of the 5-year follow-up of PROTECT AF and PREVAIL was published. It summarized a total of 4343 patient-years of follow-up. In general the stroke prevention was comparable in both groups with an additional reduction in hemorrhagic stroke and mortality [20]. The rate of ischemic stroke was numerically higher with LAAC, but it did not reach statistical significance.
The Continued Access to PROTECT AF (CAP) and Continued Access to PREVAIL (CAP2) were two registries to evaluate the safety and efficacy of the Watchman device after a longer follow-up period [21]. Holmes et al. performed a meta-analysis of two randomized trials and their respective registries. This patient-level meta-analysis included 2406 patients with a mean follow-up of 2.69 years. It showed that patients receiving the Watchman device had significantly lower rates of hemorrhagic stroke (hazard ratio [HR] = 0.22; p = 0.004), cardiovascular/unexplained death (HR = 0.48; p = 0.006), and nonprocedural bleeding (HR = 0.51; p = 0.006) in comparison to warfarin therapy [22]. The all-cause stroke or systemic embolism (SE) rate did not differ significantly between the two groups.
Although the PROTECT AF and PREVAIL trials showed good safety and efficacy of LAAC, both enrolled patients eligible for OAC therapy. The ASAP (ASA Plavix Feasibility Study with Watchman Left Atrial Appendage Closure Technology) registry was the first study to evaluate the safety and efficacy of LAAC with the Watchman device in patients unsuitable for OAC therapy [23]. This was a prospective, observational study that included 150 patients who underwent the LAAC procedure followed by 6 months of DAPT and aspirin alone thereafter. At 1 year, the rate of all-cause stroke and SE, ischemic stroke and hemorrhagic stroke was 2.3%, 1.7%, and 0.6%, respectively. The mean CHADS2 score was 2.8 with the expected risk of stroke of 7.4%. Thus, there were 77% fewer ischemic strokes than expected. The incidence of device-related thrombus (DRT) was 4% (mean: 164 ±135 days after implant), which was similar to the 3.7% reported in previous studies on Watchman device implantation followed by short-term warfarin therapy.
The EWOLUTION (Registry on Watchman Outcomes in Real-Life Utilization) was a prospective, multicenter, single-arm registry that included 1020 patients undergoing Watchman implantation. The mean CHA2DS2-VASc score was 4.5 and the HAS-BLED score was 2.3. In comparison to the previously mentioned randomized trials, the population in the EWOLUTION registry had higher thromboembolic and bleeding risk. Watchman implantation succeeded in 98.5% of cases and procedure-/device-related serious adverse events within 7 days after the procedure were observed in 2.8% of patients [24]. Post-procedure therapy included warfarin in 16% of cases, DOACs in 11%, DAPT in 60%, single antiplatelet therapy (SAPT) in 7%, and no anti-thrombotic therapy at all in 6%. So, 73% of patients had contraindications to OAC therapy and were discharged on antiplatelet therapy or without any anti-thrombotic regimen. At 1-year follow-up, ischemic stroke was observed in 1.1% of patients, resulting in an 84% relative risk reduction compared with the estimated 7.2% event rate based on the CHA2DS2-VASc score [25]. DRT and nonprocedural bleeding were observed in 3.7% and 2.3%, respectively. The rate of these events did not correlate with the post-procedure anti-thrombotic regimen.
Nowadays DOACs are superior to VKA for stroke prevention in non-valvular AF. In 2020 Osmancik et al. published the results of the first randomized trial that has sought to compare DOACs with LAAC in high-risk patients with AF [26]. PRAGUE-17 was a multicenter, randomized, noninferiority trial that included 402 patients who were randomly assigned to LAAC or DOAC in a 1 : 1 ratio. It was a high-risk patient cohort with a CHA2DS2-VASc score of 4.7 ±1.5, and the bleeding risk was assessed with the HAS-BLED score as 3.0 ±0.9 and 3.1 ±0.9 in the DOAC and LAAC group, respectively. LAAC was successful in 90.0% of patients. Watchman 2.5 and Watchman FLX devices were used in 35.9% and 2.8% of cases, respectively. In the DOAC group, apixaban was most frequently used (95.5%). At a median 19.9 months of follow-up, the annual rates of the primary outcome were 10.99% with LAAC and 13.42% with DOAC (p = 0.004 for noninferiority). In the LAAC cohort major procedure-related complications occurred in 4.5% of patients. The study concluded that LAAC was noninferior to DOACs for the composite of cardioembolic events, cardiovascular death, clinically significant bleeding, or procedure-/device-related complications.
In 2020 the National Cardiovascular Data Registry LAAO presented patient, hospital, and physician characteristics and in-hospital adverse event rates for Watchman procedures in the United States. Between January 2016 and December 2018, 38 158 Watchman procedures were performed by 1318 physicians in United States with a successful deployment rate of 93% [27]. Major in-hospital adverse events occurred in 2.16% of patients including pericardial effusion requiring intervention in 1.39% and major bleeding in 1.25%, whereas stroke (0.17%) and death (0.19%) were rare.
Watchman FLX
The Watchman FLX is the current-generation device designed to improve safety, efficacy, and implantation success. Its new design aimed to simplify the deployment in a wider range of LAA morphologies, coming in five device sizes ranging from 20 mm to 35 mm that accommodate LAA ostia diameters of 15 mm to 32 mm, and a wider range of compression (10–30%) is accepted (Figure 2). It is up to 20% shorter in length compared to the first generation device, which makes it suitable for shallow LAA. Other redesign aspects include the increased number of struts to 18 for better tissue fixation and radial strength, and two rows of ‘J’-shaped anchors enhancing its stability (Figure 2). The distal end of the new device is atraumatic, allowing partial and full recapture, and an ability to use the “ball technique” to achieve safe deployment with reduced risk of procedural complications. The device also has an extended fabric for optimal sealing and endothelialization. The Watchman FLX received Conformité Européenne (CE) marked approval in March 2019 and FDA approval in July 2020 after PINNACLE FLX trial results.
The PINNACLE FLX was a prospective, nonrandomized trial designed to evaluate the safety and efficacy of the new-generation LAAC device. The study enrolled 400 AF patients to receive the Watchman FLX. After successful occluder deployment, the antithrombotic regimen consisted of a DOAC plus aspirin for 45 days, followed by DAPT for 6 months if no peri-device leak > 5 mm was observed on TEE at 45 days. Afterwards, monotherapy with aspirin was prescribed. The trial showed a high implant success rate of 98.8%. The primary safety endpoint, defined as all-cause death, ischemic stroke, systemic embolism, and procedure-related adverse events occurring within 7 days after the procedure or by hospital discharge, was observed in only 0.5% of cases. The primary efficacy endpoint, defined as peri-device leak ≤ 5 mm in control TEE, was achieved in 100% of cases at 1-year follow-up. Moreover, at 1-year follow-up 2.6% of patients experienced ischemic stroke and 7.9% had serious bleeding complications. DRT was noted in 7 patients, with embolic events in 2 patients. Of importance, no device embolization was observed [28].
Those favorable data were supported by another prospective, multicenter registry that enrolled 165 patients in Europe. Successful deployment was achieved in all patients. The procedural related complication rate was low at 1.8% and consisted of 2 access-related complications and 1 pericardial effusion. No peri-procedural strokes, deaths, or device embolizations occurred. During a median follow-up of 55 days there were 6 bleeding complications (4.8%), 1 (0.8%) patient had an ischemic stroke, and 1 (0.8%) died. Imaging follow-up revealed 1 peri-device leak ≥ 5 mm and 7 cases of DRT (4.7%). No late device embolization was observed [29].
Lastly, the recently published FLXibility Post-Approval Study evaluated the Watchman FLX device in a commercial clinical setting. It was a prospective, observational, multicenter, single-arm study that enrolled 301 patients. One patient did not meet eligible criteria, so the implant population consisted of 300 patients. The device was successfully implanted in 99.0% of patients. Neither death nor stroke occurred within 7 days after the procedure. Three patients had pericardial effusions requiring intervention on the day of the procedure, all resolved successfully and there were no additional pericardial effusions through 1 year of follow-up. One device embolization occurred and resulted in death at 13 days after the procedure. Most patients were discharged on DAPT (87.3%), then on SAPT (7.0%) or DOAC (4.7%). The first follow-up was done between 45 and 120 days, and at that time a complete seal was observed in 88.2%, 9.5% had a leak < 3 mm, 2.4% had a leak ≥ 3 mm to ≤ 5 mm, and no > 5 mm leak was observed. The final, 1-year follow-up was performed in 93.3% of cases. Most patients were on SAPT (60.5%), followed by DAPT (21.4%), and no antiplatelet or OAC (12.1%). The all-cause mortality rate was 10.8%. Stroke occurred in 2% of cases, all of which were non-fatal strokes, and half of them were non-disabling. DRT was observed in 2.4% of patients through 1 year and all were detected at routine follow-up imaging [30].
The Watchman FLX is currently being evaluated in the ongoing CHAMPION-AF trial [31]. This is the first large, prospective, randomized, controlled trial whose primary objective is to determine whether LAAC with the WATCHMAN FLX device is a reasonable alternative to non-vitamin K oral anticoagulants in patients with non-valvular AF. The patients are randomized to the Watchman FLX or DOAC in a 1 : 1 allocation. Clinical follow-up visits are scheduled at 3 and 12 months, and then annually for 5 years. The primary outcome measures at 3 years of follow-up are Watchman FLX non-inferiority for the occurrence of stroke, cardiovascular death, and systemic embolism, and Watchman FLX superiority for non-procedural bleeding (International Society on Thrombosis and Haemostasis major bleeding and clinically relevant non-major bleeding). The estimated primary completion date is December 2027.
LAAC procedure
The pre-procedural use of TEE or CT is very important for optimal patient and device selection. This multimodality imaging allows to exclude LAA thrombus, determine dimensions and morphology of the LAA, and establish whether the procedure is feasible.
Precise assessment of LAA morphology is performed by 2-dimensional and 3-dimensional TEE. Transesophageal echocardiography can detect an LAA thrombus with high sensitivity (92%) and specificity (98%). Its negative predictive value reaches 100% and its positive predictive value is 86% [32]. The LAA is assessed at 0, 45, 90, and 135° to obtain its measurements and to establish the landing zone, maximum diameter, and depth.
A multidetector CT provides an accurate anatomical evaluation of the LAA and the perimeter, maximum and minimum diameter of the landing zone can be measured. Moreover, LAA thrombus can be excluded using CT imaging. It has a high sensitivity of 96% and a high negative predictive value of 100% for the assessment of thrombotic complications within the LAA [33]. Saw et al. demonstrated that CT provides the largest measurements, followed by TEE and fluoroscopy, concluding that CT more accurately assesses the true LAA dimensions [34].
The procedure is performed under general anesthesia or conscious sedation and guided by fluoroscopy and TEE or intracardiac echocardiography. The delivery sheath is introduced via the right femoral vein. The trans-septal puncture (TSP) should be performed in the postero-inferior segment of the fossa ovalis. The procedure is performed under adequate anticoagulation with unfractionated heparin (UFH) with activated clotting time > 250 s. Once trans-septal access is done, the trans-septal puncture sheath is swapped to the 14-Fr. device access sheath. The pigtail catheter is introduced into the LAA, through which the contrast is injected and angiographic projections of LAA are recorded. The Watchman device is advanced into the tip of the access sheath and the device is deployed under fluoroscopy and TEE guidance. The Watchman FLX is deployed by forming “a ball” (Figure 2) and either unsheathing while maintaining the position of the ball, or advancing the device distally out of the sheath until it is fully deployed. A combination of both of these techniques is possible. Too proximal position of the Watchman FLX can be corrected with the partial recapture thanks to the ball technique implantation. Before the device is released, the “PASS criteria” need to be fulfilled. P: optimal position of the device, A: checking anchor to the LAA by tug test, S: sealing assessed with color Doppler, and S: sizing for proper compression of the device.
Anti-thrombotic therapy after Watchman device implantation
The optimal anti-thrombotic regimen and its duration after a successful LAAC procedure remains controversial and should be adjusted individually. To date, various antithrombotic strategies following LAAC with the Watchman device have been proposed, including VKA, DOAC, DAPT, aspirin monotherapy, or no therapy. The main aim of this strategy is to prevent thromboembolic complications, especially during the endothelialization period directly after device deployment.
After the LAAC procedure there is a vulnerable period during endothelialization, in which there is a higher risk of developing DRT. Animal studies revealed that the endothelialization process is complete within around 90 days after the procedure [35]. Based on that result and the experience on other implantable cardiac devices, the initial strategy was developed. The first randomized trials included patients eligible for OAC therapy, so the initial protocol treatment after LAAC consisted of warfarin with aspirin for 45 days, followed by DAPT for 6 months and lifelong aspirin. However, current guidelines recommend LAAC in patients who have a contraindications for OAC, so the post-procedural regimen has shifted to less aggressive alternatives.
Numerous observational studies have been performed to assess DAPT therapy as an initial regimen following the LAAC procedure, with further lifelong aspirin. In the ASAP study, patients with a contraindication to OAC received 6-month DAPT therapy; the rate of DRT was 4%, the annual incidence of ischemic stroke was 1.7%, and a 77% reduction in events compared to that expected based on the risk score was observed [23]. The EWOLUTION registry showed that DAPT is the most frequently chosen therapeutic regimen during the first 3 months after LAAC, with 60% of the individuals in the trial on that regimen, and no significant differences in DRT and stroke rate were observed within the different treatment options [24]. The propensity-match analysis by Søndergaard et al. compared antiplatelet therapy with anticoagulation therapy following LAAC with the Watchman device in the PROTECT-AF, PREVAIL, ASAP, and EWOLUTION trials, and concluded that there were no significant differences in the incidence of major bleeding or thromboembolism between these two strategies [36]. However, DRT was more often observed in the antiplatelet therapy group, although no thromboembolic events were reported. The other trial was a meta-analysis of 12,326 patients comparing short-term OAC with antiplatelet therapy [37]. No difference in the occurrence of stroke, major bleeding, DRT, or all-cause mortality was found. Finally, our analysis of 90 patients who underwent successful Watchman 2.5 implantation in our department showed that DAPT seems to be a safe and effective regimen within 3 months after device implantation [38].
Single antiplatelet therapy and no-therapy are also used, but these regimens have a smaller amount of supporting data. Korsholm et al. published their single-center data of 110 patients who were at high risk of bleeding and were predominantly treated with SAPT [39]. A low annual major bleeding rate of 3.8% was observed, so the annual risk of major bleeding was reduced by 57%. Moreover, there was no increase in the incidence of DRT or stroke at 1 year, with a rate of 1.9% and 2.3%, respectively. Favorable results were also presented in a multicenter registry that included 600 patients allocated to SAPT (n = 280) or DAPT (n = 330) at the operator’s discretion [40]. In the SAPT group a significant reduction of major bleeding was observed (2.9% vs. 6.7%, p = 0.04), without differences in major cardiovascular events or DRT at 1 year. The other multicenter registry of 469 patients, of whom 36.2% were treated with SAPT, showed a high rate of DRT (7.2%) [41]. The multivariate analysis showed that OAC or DAPT acts as protective factor against DRT.
Finally, no therapy after Watchman device implantation has been poorly studied. This regimen can be considered in patients who are at extremely high risk of bleeding and cannot tolerate even short-term SAPT [42]. Alternatively, in this population epicardial LAA closure should be considered.
To conclude, the current data provide some reassurance on the use of DAPT after the LAAC procedure in patients who are not suitable for OAC therapy. The current recommendations from the 2020 European Society of Cardiology Guidelines on AF regarding the antithrombotic regimen after LAAC are presented in Figure 3. However, the choice of the post-procedural therapy should be individualized.
Device-related thrombus (DRT)
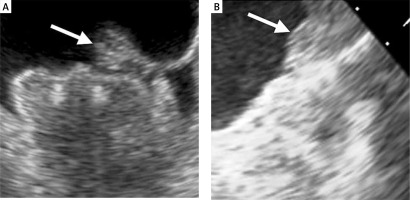
DRT is one of the most concerning complications after successful Watchman device implantation that can be detected by TEE (Figure 4) or CT. In the PROTECT-AF trial the rate of DRT was 4.2% [17]. However, in 2018 Dukkipati et al. published cumulative data regarding DRT incidence in PROTECT AF, PREVAIL, and their continuous access registries [43]. After a mean follow-up time of 4.1 years, the rate of DRT was 3.74%. The analysis of patients with DRT revealed that they were at higher thromboembolic risk assessed with the CHA2DS2-VASc score, were more likely in permanent atrial fibrillation, had a history of TIA or stroke, larger LAA, and had lower ejection fraction (EF). The risk of stroke or systemic embolism was 3.55-fold higher in patients with DRT. In the EWOLUTION registry, at 2-year follow-up DRT was observed in 34 (4.1%) cases among 835 patients with imaging of the LAA after a median time of 54 days (IQR: 41–111 days) [44]. Thirty-one incidents of DRT were observed at the first follow-up imaging usually within 90 days after the procedure. DRT occurred irrespective of the type of the post-LAAC antithrombotic regimen. Patients with DRT more often had non-paroxysmal AF, evidence of spontaneous echo contrast, and a larger ostium diameter of the LAA [45]. Overall, no significant differences in rate of thromboembolic events were observed in patients with or without DRT (1.7 vs. 2.2%/year, p = 0.8). In a prospective registry with the second-generation Watchman FLX device, at 1 year DRT was detected in 7 (1.7%) patients with 4 cases discovered during scheduled follow-up visits [28]. In the real-world registry of Watchman FLX, DRT was observed in 2.4% of cases through 1 year of follow-up and all were detected during routine imaging [30].
Figure 4
Device-related thrombus on Watchman 2.5 device. The arrow indicates a thrombus on the surface of the implanted device
There are several procedural- and patient-related factors that predispose to DRT. The predictors of DRT include reduced EF, a history of a thromboembolic event, or a larger orifice of the LAA [37, 42, 43]. Furthermore, a deep Watchman device implantation, leaving a large volume of uncovered appendage (“neo-appendage”), and a lack of left upper pulmonary vein ridge coverage are linked with increased risk of DRT. In 2018 Pracon et al. reported that DRT was more often observed in patients with deep device implantation [46].
To date, management of DRT remains a clinical issue. Most data suggest the use of antithrombotic therapy with heparin or OAC [41]. However, this approach still raises concerns, mainly about the risk of bleeding. Most patients referred for LAAC have contraindications to OAC therapy, so the initiation of OAC significantly increases the risk of bleeding, but on the other hand there is a high risk of embolism because of DRT presence. Secondly, up to 30% of DRTs may persist despite proper anticoagulation therapy; thus, these patients experience higher morbidity and mortality [47]. Moreover, the recurrence rate of DRT while the patient is still on anticoagulation is 35%, while the recurrence rate increases to 50% when the OAC therapy is abandoned [48]. Another treatment option for DRT is thrombus aspiration. Its feasibility has been reported, but the safety and efficacy of this approach have not been established and there are no data at this time to support routine use of aspiration thrombectomy over medical therapy [49].
Further studies are required to investigate the post-procedural optimal antithrombotic regimen to minimize the risk of DRT. This approach should take into account the patient’s thromboembolic and bleeding risk, and also predictors of thrombus formation.
Peri-device leak
The Watchman device has a standard circular shape, while the ostium of the LAA is typically elliptical and shows considerable interpatient variability. This mismatch between the shape of the device and the LAA can cause incomplete LAA occlusion with increased risk of residual peri-device leak (PDL). Peri-device leak has been defined as residual flow of any size around the implanted device which is detected with TEE. The PDL around the Watchman device is defined as minor when it is < 3 mm, moderate at 3–5 mm, and significant when > 5 mm.
In the PROTECT AF study, any PDL was observed in 40.9% of cases at 45 days, while at 1 year it had decreased to 32.1% [50]. Peri-device leaks > 3 mm were present in 13.3% at 45 days and in 11.8% at 1 year. The new generation Watchman FLX was evaluated in the PINNACLE registry, in which the data were assessed with the echocardiography core lab and lower incidence of PDL with the Watchman FLX device was revealed [28]. Any PDL was observed in 17.4% of patients at 45 days, and in 10.5% at 1 year. The recent report from the SURPASS study, which investigated the Watchman FLX in a real-world setting, showed an even lower rate of PDL [51]. In 2022 the SURPASS analysis included data from more than 16,000 patients and showed that after Watchman FLX implantation, 95.3% of the patients had no peri-device leak, and 99.1% had no leak or had a leak of < 3 mm. At 45 days of follow-up, these rates decreased to 83% and 95% respectively.
It has been debated whether the presence of PDL following Watchman device implantation is associated with increased thromboembolic risk. To date we know that leakage after surgical LAA ligation is connected with increased thromboembolic complications [52]. The rate of stroke or systemic embolism is relatively low after percutaneous LAAC, so assessing the independent impact of PDL on thromboembolic outcomes is difficult and would require a very large sample size. Moreover, patients with significant leakage of > 5 mm are recommended to receive anticoagulation therapy [53], and hence the evaluation of the influence of the residual peri-device leak on thromboembolic outcomes may be disturbed by treatment bias. In the PROTECT AF study the PDL size was not associated with an increased risk of thromboembolic complications [50]. However, this study was underpowered in this regard. A recent analysis combined PROTECT AF, PREVAIL, and CAP2 studies to evaluate the impact of PDL in a population of 1054 patients who underwent LAAC with the Watchman 2.5 device [54]. The study showed that 39.8% and 28.4% of patients had a peri-device leak at the 45-day and 1-year TEE, respectively. The presence of PDL ≤ 5 at 1 year, but not at 45 days, was associated with an increased 5-year risk of ischemic stroke or systemic embolism (HR = 1.94; 95% CI: 1.15–3.29; p = 0.014), largely driven by an increase in nondisabling stroke (HR = 1.97; 95% CI: 1.03–3.78; p = 0.04), while disabling or fatal stroke rates were similar (HR = 0.69; 95% CI: 0.19–2.46; p = 0.56).
To date, no ideal management of PDL has been offered. The introduction of pre-procedural CT to obtain optimal sizing and better alignment with the LAA has been advocated to achieve better sealing [55]. In the case of a small PDL < 5 mm, a watchful waiting strategy has been proposed due to a documented regression of small leaks in 20% to 40% of patients [28, 50]. A significant PDL should be managed with anticoagulation therapy. Another option is closure of the PDL with plugs or a coil, and this approach has been reported in several case series [56, 57]. These studies revealed that complete or near-complete PDL occlusion is feasible in most patients with a low rate of complications, but long-term outcomes are still unknown [58].
Conclusions
Percutaneous LAAC with the Watchman device is an attractive treatment alternative for stroke prevention in the growing population of patients who are not good candidates for OAC therapy. As the new device is introduced into clinical practice and operator experience grows, the LAAC procedure becomes an increasingly safe and effective treatment option. However, there are still some concerns regarding long-term adverse events (i.e. DRT and PDL) and optimal post-procedural patient management. Further studies are necessary to optimize patient selection and the post-LAAC antithrombotic regimen, and determine the long-term benefit of LAAC.










