Dear Editor,
Perioperative bleeding is a common complication in patients undergoing orthopedic surgery and is associated with increased morbidity and mortality [1, 2]. Tranexamic acid (TXA) is an antifibrinolytic drug which reduces surgical blood loss and allogeneic transfusion after total knee arthroplasty (TKA) and total hip arthroplasty (THA) [3–5] and can be effectively administered orally, topically, or intravenously [6]. Administration of TXA during TKA and THA is strongly recommended in clinical practice guidelines issued by the American Association of Hip and Knee Surgeons, American Society of Regional Anesthesia and Pain Medicine, American Academy of Ortho-pedic Surgeons, Hip Society, and Knee Society as an effective strategy for reducing blood loss and the need for transfusion [7]. This study aimed to determine the utilization of TXA by Polish anesthesiologists and orthopedists during TKA and THA procedures. The analysis was conducted to assess whether the practice of Polish anesthesiologists and orthopedists is consistent with the current guidelines.
It was a survey-based study carried out on-line (LimeSurvey application, version 3.26.1+210427) in two rounds: 17 May – 30 July 2021 and 3 August – 24 August 2021. The questionnaire was created after analyzing the available literature specifying the key elements of perioperative management. Details concerning the questionnaire can be found elsewhere [8]. There was one single-choice question concerning perioperative TXA administration and the possible answers included: (a) none, (b) locally, (c) orally, (d) intravenously, (e) leave decision to the anesthesiologist/orthopedist. A list of healthcare institutions was obtained from the National Health Fund (NFZ) data thanks to collaboration with the National Centre for Quality Assessment in Healthcare. The survey was sent to all healthcare institutions in Poland performing elective TKA and THA procedures in adult patients. Due to the character of the study, there was no need for patient informed consent. The study was approved by the Bioethics Committee of the Jagiellonian University (No. 1072.6120.259.2020 of September 24, 2020).
The categorical variables were presented as counts and proportions and compared using the χ2 test or Fisher exact test. A two-tailed P-value < 0.05 was considered significant. Statistical analyses were performed with R Studio, version 4.1.0.
The survey was completed by 238 anesthesiologists and 131 orthopedists practicing in 112 centers in Poland. TXA was used by 81/238 (34%) anesthesiologists and 79/131 (60.3%) orthopedists. Anesthesiologists preferred intravenous (98.8%) over oral administration (1.2%). The most common routes of TXA application reported by the orthopedists were intravenous (83.5%) followed by topical (13.9%) and oral (2.5%). 86/238 (36.1%) anesthe-siologists leave the decision regarding the TXA administration to orthopedists. No intraprocedural administration of TXA was reported by 31/131 (23.7%) orthopedists and 65/238 (27.3%) anesthesiologists. The attitude of anesthesiologists and orthopedists to the TXA administration is presented in Figure 1. There were no statistically significant differences among respondents according to the different types of hospital (university vs. non-university), volumes of the hospital (number of beds), or years of clinical experience.
FIGURE 1
Attitude of orthopedists and anesthesiologists to tranexamic acid administration during total knee and hip arthroplasty
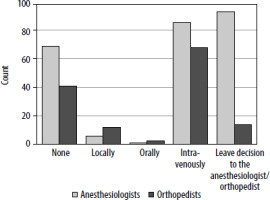
In this survey-based study describing the real-life perioperative TXA utilization in TKA and THA in Poland, we found that TXA is administered by a third of anesthesiologists and 60% of orthopedists and that the preferred administration route was intravenous.
Even though the current guidelines clearly state that perioperative TXA administration in total joint arthroplasty reduces bleeding and need for transfusion, our study shows that approxima-tely a quarter of Polish anesthe-siologists and orthopedic surgeons do not use it in this clinical context [7]. In a similar questionnaire-based study performed in England in 2014, 38% of anesthetic leads of participating hospitals reported routine TXA admi-nistration during hip and knee arthroplasty [9].
Recently published results of the Perioperative Ischemic Evaluation-3 (POISE-3) trial, which comprised 9535 patients undergoing noncardiac surgery, showed (despite not reaching the noninferiority threshold) that the likelihood of composite bleeding outcome events (such as a composite of life-threatening bleeding, major bleeding, and bleeding into a critical organ) was lower with TXA than with placebo [10]. Notably, 2146 patients enrolled in the POISE-3 study underwent orthopedic surgery, and it is likely that the POISE-3 trial results will strengthen the prior recommendations of using TXA in this group of patients.
Furthermore, the risk-benefit profile of TXA usage favors the benefit side. There were no differences in the POISE-3 trial in terms of safety, and a recently published large retrospective analysis, which included 26,808 patients with a history of coronary artery disease or coronary stents undergoing total joint arthroplasty, showed no increased risk of venous thrombosis or myocardial infarction in patients receiving TXA [11].
To sum up, compared to a previous report, our study suggests growing TXA utilization during TKA and THA (38% of cases in 2014 and 75% in our study). However, there is still room for improvement, as 25% of patients do not receive TXA despite strong recommendation for this intervention. Thus, further education regarding TXA utilization in orthopedic surgery is needed.
Out analysis is limited by the lack of precise data about the exact admi-nistration schedule of TXA (e.g. dosage or repeatable application). Moreover, we did not analyze potential cases of patients’ contraindications to TXA administration.




