Introduction
Eye tumours can cause symptoms such as a decrease or loss of vision and inflamed and painful eye, but they can also be a cause of death due to local spread or metastatic disease. Uveal melanoma (UM) is considered the most aggressive intraocular tumour in adults: up to 50% of patients die within 15 years after enucleation or other therapeutic methods [1]. Uveal melanoma mainly metastasizes in other regions of the body such as liver, lung, and kidney.
Treatment of ocular malignancies aims to preserve the eye with the best possible vision, while reducing the risk of death.
In the past years, enucleation represented the standard therapy [2], but in recent times most primary UMs have been treated conservatively with radiotherapy, as well as when enucleation remains necessary in some cases of very large tumours [3]. All radiotherapy modalities show promising results in globe preservation and vision sparing.
In 2001, the Collaborative Ocular Melanoma Study (COMS) showed that globe-preserving brachytherapy (BT) for UM is safe and effective, demonstrating no survival difference with enucleation [4]. Following these results, nowadays it is common practice to propose BT for tumours without extrascleral extension, while in cases of very large tumour or for particular locations (lesions close to the optic nerve or in the posterior pole of the globe) other conservative techniques of fractionated stereotactic radiotherapy have been suggested [5, 6]. Hypofractionated stereotactic body radiotherapy (SBRT) is administered using a few large fractions or a single large fraction. Stereotactic radiosurgery (SRS) provides the maximum dose to tumour tissue and minimizes the irradiation of healthy tissues [7]. Devices used for SRS include the Gamma Knife, CyberKnife, and linear accelerators (LINAC). Hypofractionated radiation modalities could enhance tumour control and use imaging and technological advances to reduce toxicity; these approaches have been widely adopted because it has been shown that UM appears to be relatively radio-resistant to hyperfractionated radiotherapy [8].
In the present case, we report the outcome of a patient with locally recurrent UM, previously treated with BT, using a second personalized globe-sparing radiotherapy approach.
Case report
In June 2020, a 78-year-old male patient was clinically examined by the Ophthalmologists of the Ophthalmic Surgery Division of the Azienda Ospedaliera Universitaria Pisana with diplopia, suspected UM in the right eye, and an evolving cataract in both eyes. The best-corrected visual acuity was 8/10 in the right eye. At the ophthalmological evaluation with biomicroscopy, ophthalmoscopy, and standardized (A-scan and B-scan) ultrasonography, a lesion in the right eye at 6–7 hours of about 5 mm thickness with internal lacunar areas, approximately 7 mm away from the limbus, was observed. The patient underwent ruthenium plaque BT at a total dose of 110 Gy to the apex of the tumour. Twelve months after BT, the lesion volume appeared to be reduced (maximum height 2.2 mm, maximum diameter 6 mm), and a complete halo of cicatricial atrophy surrounded the treated retinal area.
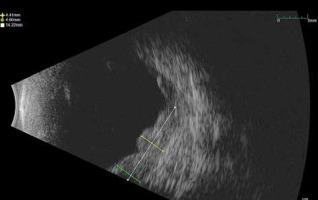
In September 2021, a new multi-nodular lesion with a satellite exudative detachment was observed in the lower and temporal sectors 7–10 hours. At the B-scan the lesion had a maximum thickness of 4.6 mm (Fig. 1). A total body computed tomography (CT) was performed to determine tumour restaging and to exclude distant metastases at this time. Subsequently, in a multidisciplinary discussion and in consideration of the non-feasibility of a new plaque placement, a single fraction SRS was scheduled. A computed tomography simulation with a GE Speed RT 16 CT Simulator was performed, using an immobilizing thermoplastic mask and an eye fixation system to ensure satisfactory reproducibility of the eye position with respect to the simulation CT images. Our device consisted of a blinking light diode on which the patient could focus the gaze. A camera system was used to monitor the eye movements of the patient.
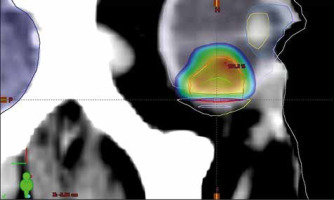
The site of the previous irradiation was outlined on the CT study. The clinical target volume was identified, and an isotropic margin of 2 mm was used as expansion to the planned target volume (PTV). The prescribed dose to the PTV was of 27 Gy in a single fraction, with the constraint of not less than 95% prescribed dose on the 95% PTV. The maximum dose limit on the previously irradiated volume was of 24 Gy. The planning doses to critical structures were below 8 Gy for the optic nerve and the optic disc, and 10 Gy for the eyelids and anterior segment of the eye (Fig. 2).
Fig. 2
Reconstructed computed tomography sagittal section and the relative stereotactic radiosurgery dose distribution; the visible contoured structures are the lens (yellow, top), the anterior chamber (light blue), the whole right eye (grey), the previously irradiated volume (yellow, bottom), the body profile (white), the brain (dark blue), the clinical target volume (green), the restricted planned target volume (PTV) to 27 Gy (red), and the PTV portion irradiated to 20 Gy (dark red). Percentage dose is shown in colour wash modality, from the local maximum of 101.2% (hottest colour) to 10% (coldest colour)
Assuming a conventional value of 10 Gy for the UM α/β ratio, we calculated a biological effective dose (BED) ≈ 100 Gy.
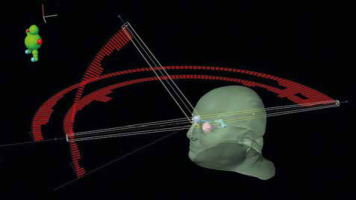
Stereotactic radiosurgery was delivered with a True Beam LINAC (Varian Medical Systems, Palo Alto, US), and volumetric modulated arc therapy (VMAT) with the rapid arc technique, using 6MV flattener free field (FFF) beams, in October 2021 (Fig. 3).
Fig. 3
Stereotactic radiosurgery irradiation pattern with volumetric modulated arc therapy and rapid arc technique. The external body surface and major organs at risk have been reconstructed, and the photon beam intensity along each arc is reported (red bins)
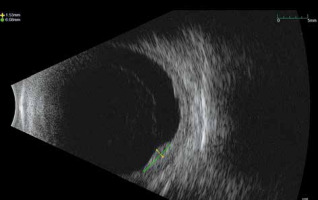
The time interval between the 2 treatments was 15 months. Twenty months after recurrence, US examination showed a tumour volume reduction and a re-absorption of the initial exudative detachment (Fig. 4). No metastases were detected on follow-up examinations (CT total body/PET once a year and liver US every 6 months). Optical coherence tomography showed macular pucker without signs of retinal oedema in the right eye and a physiologic macula with retinal pigment epithelium (EPR) anomalies in the left eye. No severe acute or late toxicity events were observed due to the retreatment.
Discussion
Since the publication of the COMS study, conservative treatment has been a standard of care for UM; the COMS trial also showed that radiation therapy offered a potential for local control and visual preservation [4]. In a large study on 650 patients with a follow-up of 60 months after 125I BT, the local failure rate was 10.3% [9].
Several studies showed that patients with local recurrence may have an increased risk of metastasis and decreased survival [10, 11]. Because there is no generally shared consent for retreatment, the treatment approach remains a single case decision and depends on the extent and the location of the recurrence [12, 13].
Re-treatment options for those patients are limited: some local recurrences can be treated with focal laser, cryotherapy, or laser hyperthermia, but the majority undergo enucleation [14]. Radiotherapy could also be effective in the treatment of recurrences after primary treatment, although few experiences have been reported.
Damato et al. showed that proton beam radiotherapy can be administered as primary treatment of UM, as well as salvage therapy for recurrent UM [15]. Riechardt et al. evaluated 48 patients with local recurrence of UM after primary treatment with BT, transpupillary thermotherapy, proton beam therapy, laser photocoagulation, CyberKnife radiation, or photodynamic therapy, who underwent proton beam salvage therapy. This study concluded that proton beam salvage treatment resulted in high local tumour control in recurrent UM, especially if the primary therapy was transpupillary thermotherapy or plaque BT. Preservation of the globe was possible in most patients [16].
Also, BT [17] and Gamma Knife radiosurgery [18] have been employed to retreat recurrent UM. Tagliaferri et al. [17] suggested that in selected cases treated with primary plaque BT, especially in the presence of marginal local recurrence, a customized re-treatment with plaque BT may offer high probability of tumour control and organ preservation but worsening of visual acuity. Sorour et al. reported the first case of successful of GKR salvage therapy after plaque BT primary treatment failure in a choroidal melanoma. At 3 years of follow-up after GKR, the patients showed a preserved visual acuity and no evidence of systemic diseases [18].
The dose prescribed to a radiotherapy treatment must be decided considering the sensitivity to the irradiation of the specific tumour histology. Van den Aardweg et al. [19] reported their in-vitro determinations of several radiobiological parameters for different clonogenic lines of primary and metastatic UM cells. They found that the α/β ratio was in the range of 3.5–84.5 Gy for primary tumours and of 4.7–14.1 Gy for metastatic lines. These α /β values were strongly dependent on the clonogenic samples and could not be straightforwardly applied to the clinical practice.
Basing on the conclusions of the van den Aardweg study, Gagne et al. [20] excluded the value of α /β= 84.5 Gy, which should correspond to a very radiosensitive tissue, and in their paper they assumed α /β = 11.5 Gy. Their conclusions were applied to the analysis of dose distributions in 125I BT treatments.
In the present study, we adopted a conventional value of 10 Gy for the α /β ratio, because it is close to the average of in-vitro experimental determinations of Van den Aardweg and is widely employed in BED calculations.
To prescribe the dose to the SRS treatment, we analysed other experiences of photon stereotactic radiosurgery reported in the literature. Among all, we mention the paper of Diekmann et al. [21], who reported a series of 158 patients treated for UM with photons in 5 fractions of 10 Gy (total dose, 50 Gy). They stated that the applied dose of 60 Gy in 5 fractions was not different from the standard doses of 54–70 CGE applied in 4 to 5 fractions by proton studies. They did not observe significant differences with proton studies when looking at the total number of post-radiotherapy enucleations. Following this experience, we calculated (assuming an α /β ratio of 10) that in the work of Diekmann the BED was 100.
The previous experiences of Furdova et al. [22] and Laliscia et al. [6] in primary UM treated with LINAC-based SRS (35 Gy and 27 Gy, respectively) suggested that eye-globe preserving treatment with LINAC-based stereotactic irradiation is feasible and well-tolerated in patients with large and unfavourably located uveal melanoma.
So, in this case, considering that a single fraction could help reduce set-up errors that can add up in multi- fractionation, we prescribed a single fraction of 27 Gy, for which we calculated a BED ≈ 100. We concluded that this treatment schedule could be as effective on the tumour as the Diekmann scheme. Photon beam SRS was used as an eye preserving salvage therapy after local recurrence of ocular melanoma. With 35 months of follow-up after initial diagnosis there is no clinical evidence of local recurrence and systemic metastases. Moreover, based on Milano et al. [23] pooled analysis, patients with prior radiotherapy appeared to be at a > 10-fold greater risk of radiation- induced optic nerve/chiasm injury, after one fraction or multifraction SBRT. But the real increased risk from prior radiotherapy is limited by the small number of reported events and the multitude of variables. In our case, 20 months after SRS, we only observed a cataract progression in both eyes and also an irido-lenticular synechia and exotropia in the treated eye; the best corrected visual acuity was 4/10 in the treated eye
The clinical evidence suggested that the lesion was under control in both treated sites.