Spinal cord ischemia (SCI) after cardiopulmonary bypass (CPB) is a rare neurologic complication [1]. Causal mechanisms include cardiac arrest, non-pulsatile blood flow, emboli, loss of spinal cord collateral circulation and loss of cerebral autoregulation [2]. The reported incidence for paraplegia is 2–10%, while the incidence for paraplegia is 0–10% after open repair of thoracoabdominal aneurysms [3]. Paraplegia due to SCI following CPB for cardiac surgery not involving the aorta is an extremely rare phenomenon [1]. Nevertheless, it remains a catastrophic cause of disability. Surgeons, anesthesiologists and intensivists should be aware of it in order to deliver optimal patient care.
We describe a case of postoperative paraplegia in a patient who underwent isolated tricuspid valve replacement with beating heart CPB.
An 81-year-old man with atherosclerosis and atrial fibrillation visited the emergency department reporting shortness of breath for a few hours. Twenty days before his visit, he had undergone transcatheter edge-to-edge repair (TEER) of the tricuspid valve performed with the TriClip Repair System [4] due to severe tricuspid regurgitation. The patient’s past surgical history included a mechanical aortic valve placement along with ascending aorta replacement and double coronary artery bypass grafting (CABG) 12 years ago. The left internal mammary artery (LIMA) was anastomosed to the left anterior descending (LAD) artery and a saphenous vein graft (SVG) was anastomosed to the obtuse marginal artery (OMA).
Upon clinical assessment, the patient exhibited anasarca, visibly distended jugular veins, and clinical signs suggestive of respiratory failure, indicating right heart failure. Electrocardiography showed atrial fibrillation without ST elevation, depression or T wave flattening or inversion. Troponin levels were not elevated. Initial transthoracic echocardiography (TTE) demonstrated a left ventricular ejection fraction of 60%, indicating preserved left ventricular contractility. The right ventricle appeared normal in size but exhibited marginally impaired contractility, with a tricuspid annular plane systolic excursion (TAPSE) of 16 mm. Both atria were enlarged. A wavy structure, which could be attributed to a detached clip, appeared on the flail septal leaflet of the tricuspid valve, which presented significant regurgitation. These findings indicated a failed TriClip repair. The computed tomography (CT) scan showed diffuse ground glass opacities consistent with pulmonary edema. Initially, the patient was treated with diuretics and 16 days later he was transferred to the cardiothoracic surgery department. As he had been treated with acenocoumarol, he was switched to therapeutic dosage of low molecular weight heparin (LMWH) 5 days prior to surgery according to international guidelines [5].
Twenty-one days after the initial presentation, the patient underwent tricuspid valve replacement using a bioprosthetic valve via a right thoracotomy approach. Preoperatively, he was hemodynamically stable, with systolic blood pressure (SBP) at 136 mm Hg, mean arterial pressure (MAP) at 85 mm Hg and a cardiac index equal to 2.4 l/min/m2. As he was at high risk for complications including ischemic injury, neurological injuries, and severe hemodynamic instability, beating heart CPB was preferred, with a total duration of 154 minutes. A left femoral arterial line was used to demonstrate the arterial pressure of the lower body part. The left subclavian artery and the right femoral vein were cannulated for CPB. Coagulation was based on a standard heparin protocol. Mild [2] hypothermia was maintained during surgery with a mean temperature of 35°C. Flow rate was maintained between 2.2 and 2.4 l/min/m2. Mean arterial pressure was maintained between 60 and 70 mm Hg for most of the procedure. However, there was a brief episode of hypotension lasting less than 3 minutes where MAP dropped below 60 mm Hg but remained above 40 mm Hg. The cardiac index ranged from 2.2 to 2.4 l/min/m2. Noradrenaline at 0.3 µg/kg/min was administered to achieve target MAP. Intraoperative transesophageal echocardiography (TEE) did not reveal any left atrial appendage thrombus or any AVR-associated masses. No intracardiac shunt or patent foramen ovale (PFO) was detected. Minor aortic atheroma was found (grade III, less than 5 mm) [6] located at the minor curve of the aortic arch. The patient was administered one unit of packed red blood cells due to low levels of hemoglobin (less than 7 g/dl) intraoperatively.
Postoperatively, the patient was transferred to the intensive care unit (ICU), and extubated 18 hours later, on the next day. During the first 24 hours in the ICU, he remained in need of vasopressors (noradrenaline at 0.3–0.4 µg/kg/min and vasopressin at 0.03 IU/min) to continuously maintain MAP over 75 mm Hg. Postoperative TTE showed that the bioprosthetic tricuspid valve replacement was functioning well without evidence of leaflet stenosis, regurgitation, or paravalvular leak. The estimated right ventricular systolic pressure was 30 mm Hg based on the tricuspid regurgitation jet velocity. However, TTE revealed worsening of right ventricular systolic function compared to preoperative assessment, with a reduced tricuspid annular plane systolic excursion (TAPSE) of 12 mm, indicating severe right ventricular dysfunction. Left ventricular ejection fraction was preserved at 60%. Levosimendan was initiated as a continuous infusion at 0.1 µg/kg/min. The patient gradually improved and was weaned off vasopressors and inotropes at approximately 40 hours after ICU admission.
Before extubation and as he regained consciousness, the patient presented bilateral weakness in both lower limbs. Upon neurological assessment, bilateral flaccid paralysis of the lower limbs and impaired sensation extending from the T10 dermatome and below were noted. Cranial nerve function was normal. No weakness or sensory deficit was detected in the upper limbs. A TEE was performed to exclude aortic dissection. Magnetic resonance imaging (MRI), as shown in Figures 1 and 2, was typical of myelopathy due to spinal cord infarction, possibly due to low flow to the Adamkiewicz artery. As MRI and TEE showed, there was no evident dissection of the artery of Adamkiewicz or of the aorta in its origin and no dissection of the aorta at any level.
Figure 1
Magnetic resonance of lumbar spine. Sagittal T2-weighted sequence demonstrates increased T2 signal, along with spinal cord expansion, in intramedullary spinal cord from T11 level to the conus medullaris reflecting spinal cord infarct (white arrow). Chronic compression fracture of L2 vertebral body, without edema, is recognized as an incidental finding
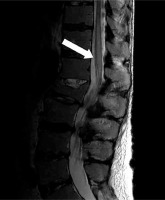
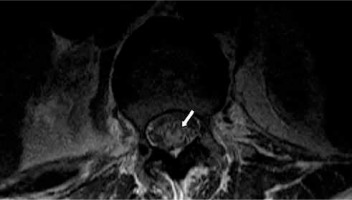
Figure 2
Magnetic resonance of lumbar spine. Axial T2-weighted sequence (T12 level) demonstrates intramedullary increased T2 signal (owl’s eyes sign – white arrow)
As per neurology recommendations, hemodynamic augmentation was provided to maintain a target MAP greater than 75 mm Hg along with intensive physical therapy. On postoperative day 16 the patient presented postoperative left hemothorax and thus he underwent a second operation consisting of left thoracotomy, drainage, and evacuation of clotted blood. Subsequently, he manifested lower gastrointestinal bleeding due to which he was subjected to colonoscopy and polypectomy. Throughout his ICU stay, his neurological condition remained unchanged. On postoperative day 28, he presented acute respiratory failure along with fever. As he deteriorated rapidly, he was reintubated. He eventually exhibited an increasing need for vasopressors (noradrenaline at 0.8 µg/kg/min and vasopressin at 0.03 IU/min) as he developed severe septic shock which was treated with broad-spectrum antibiotics but caused multiple organ disorder syndrome. He never recovered from the shock and, despite medical interventions, he died in the ICU. Postmortem, no autopsy was performed to establish a definitive cause of spinal cord injury.
Regarding the anatomy of the arteries feeding the spine, the intrinsic arteries of the spinal cord can be separated into a central and a peripheral system [7]. The central system, which supplies two-thirds of the spinal cord, is derived from the anterior spinal artery. In the peripheral system, the blood flows from the two posterior spinal arteries [7]. The artery of Adamkiewicz typically enters the spinal cord on the left from T9–L1 and is the primary supply to its lower two-thirds. Through autoregulation, spinal blood flow is maintained at a constant level. When autoregulation fails, systemic hypotension may reduce perfusion and put the cord at risk, resulting in spinal cord infarction [8].
While aortic surgery is the leading cause of perioperative SCI [9, 10], non-aortic cardiovascular surgery also confers risk [1]. Proposed causal mechanisms include systemic hypoperfusion, thromboembolic events including cardiogenic embolism and hypercoagulable conditions, hemorrhagic complications such as epidural hematoma or spinal canal hemorrhage, spinal compression due to a herniated or fractured disc and aortic dissection [1]. Intra-aortic balloon pump (IABP) may also contribute to SCI in some cases [11–13]. In this specific case, malperfusion or dissection of the left vertebral artery, which arises from the left subclavian artery, could impact the anterior spinal artery and the Adamkiewicz artery, subsequently causing prolonged malperfusion of the spinal cord. A definitive causal mechanism in such cases often remains uncertain [1].
In the case presented, no spinal disc fracture, epidural hematoma, spinal canal hemorrhage or any other cause of spinal compression were observed in MRI. Neither the MRI nor the postoperative TEE showed any evidence of aortic dissection at any level. An intra-aortic balloon pump was not utilized in this patient. Regarding thromboembolic events, including atheroma plaque emboli, there was no guidewire placement into the descending thoracic aorta and no aortic plaque was dislodged. Minimal aortic atheroma was found using TEE. While it is very difficult to distinguish between embolism and thrombosis, there were no abnormal clotting or coagulation disorders intraoperatively and the patient was sufficiently, as per protocol, anticoagulated. As the patient manifested hypotension intraoperatively with MAP < 60 mm Hg, and even though it was of short duration, hypotension may have contributed to hypoperfusion injury of the spinal cord [1]. Eventually, using vasopressors, MAP levels were stabilized and, postoperatively, they were maintained continuously above 75 mm Hg. Watershed spinal cord infarction is an extremely rare entity that, following aortic or non-aortic cardiac surgery, can be caused by prolonged hypotension [14, 15]. Although we believe that hypoperfusion with systemic hypotension could have contributed to causing SCI in this case, MRI indicated Adamkiewicz artery involvement. Based on scarce literature data [14, 15], the imaging findings do not support a diagnosis of a watershed infarct as far as typical signs and vascular anatomy distribution are concerned. Thus, the radiology team could not declare findings typical of global hypoperfusion and watershed infarct.
Specifically for this case, left subclavian artery cannulation could have led to malperfusion or dissection of the left vertebral artery, eventually causing prolonged decreased flow to the Adamkiewicz artery, as described above. Absence of posterior ischemic stroke and cervical SCI can be attributed to sufficient collateral vertebrobasilar circulation and supply from the cervical arteries. Furthermore, regarding the thoracic spine, blood supply higher than the T10 level could be maintained through segmental arteries branching from the dorsal aorta. Although the post-operative left hemothorax could be an indirect indicator of left vertebral artery injury, no such evidence was found on the MRI or the CT scan. However, considering these findings, hypoperfusion of the left vertebral artery cannot be ruled out as a possible mechanism.
Treatment options for spinal cord ischemia include thrombolytic therapy, corticosteroids, hemodynamic augmentation and lumbar drain [10, 16]. Thrombolysis with intravenous tissue plasminogen activator (tPA) was contra-indicated in our case as the patient had undergone cardiac surgery in the last 24 hours [17]. A very small number of cases [18–20] in which thrombolysis was used as a treatment for spinal cord stroke have been reported, but they do not include recent cardiac surgery patients, who carry a major bleeding risk. Corticosteroids, such as high dose methyl-prednisone and dexamethasone, have been used more frequently in spinal cord ischemia but clinical evidence weighing harmful and beneficial effects is controversial in SCI related to cardiovascular surgery [16, 21]. The combination of hemodynamic augmentation with vasopressor and lumbar cerebrospinal fluid (CSF) drainage has shown promising results, although they are not the standard of care [21]. CSF drainage has been used perioperatively in high-risk patients undergoing thoracoabdominal aortic surgery to improve spinal perfusion by decreasing intraspinal pressure, thus lowering the risk of spinal ischemia [10, 21, 22]. Considering that our case did not involve aortic surgery but valve replacement, a procedure rarely associated with SCI, a lumbar CSF drain was not inserted preoperatively to prevent it. As there is little evidence regarding a lumbar drain, the neurology team’s recommendations included MAP augmentation and physical therapy [10, 21–23].
A recent review [1] summarized the clinical evidence of spinal cord ischemia after cardiac surgery not involving the aorta. In 8 cases of isolated valve replacement surgery, postoperative paraplegia was reported [24–31]. Six of them were attributed to perispinal bleeding and hematoma [25–27, 29–31], one to hypotension and loss of circulation to the spinal arteries [24] and one to undefined causes [28]. Four of them included isolated mitral valve surgery, three included aortic valve surgery and one referred to a case of mitral valve replacement and tricuspid valve annuloplasty. To our knowledge, we report the first case of postoperative paraplegia due to spinal cord infarction, following isolated tricuspid surgery.
Research is still needed in preventing neurological complications due to isolated cardiac surgery. Hypothermia and cerebrospinal fluid drainage have been recommended for high-risk repairs of descending thoracic and thoracoabdominal aortic surgery, but not for isolated cardiac surgery [32]. Furthermore, as in some cases autonomous regulation could be unbalanced due to atherosclerosis and age, permitting a higher mean arterial pressure threshold intraoperatively has been reported to prevent spinal cord injury [1]. During preoperative preparations, meticulous screening for risk factors such as atherosclerosis should always be performed to assess the overall risk of postoperative complications. Regarding subclavian artery cannulation, screening for cranio-cervical arterial anomalies and competence of the circle of Willis with preoperative MR angiography could be helpful. Subclavian artery perfusion can also be evaluated intra-operatively using TEE.
We describe the case of a patient who underwent tricuspid valve surgery, with a history of dyslipidemia and CABG along with aortic valve replacement and aorta replacement, who was recently subjected to TEER with a TriClip. However, the patient presented an extremely rare but devastating complication. We consider that hemodynamic instability intraoperatively and unbalanced autonomous regulation due to age and atherosclerosis were risk factors for postoperative SCI in this patient. Along with these comorbidities, left subclavian artery cannulation could have posed a risk for compromised flow to the left vertebral artery, although this fact could not be proven. A thromboembolic causal mechanism appears to be less likely, but as an autopsy was never performed, this scenario cannot be excluded. Methods to prevent the occurrence of paraplegia following cardiac surgery not involving the aorta should be routinely adopted. Nevertheless, a case like this suggests that cardiothoracic surgeons, anesthesiologists and intensivists should be familiar with this complication and maintain a vigilant approach to detect this entity early.






