Procalcitonin (PCT) is an early biochemical marker of severe bacterial infection, and its serum level rises within a few hours after the onset of sepsis [1–3]. Numerous studies have confirmed its role in the diagnosis of sepsis, especially sepsis with positive blood cultures (BC) [4–6]. Although non-infectious postoperative complications can induce PCT synthesis, PCT is effective in differentiating sepsis and systemic inflammatory response of non-infectious origin [7]. The level of PCT can rise above 0.5 mg L–1 in the first few days after surgery, but in patients with postoperative infectious complications this level is significantly higher. On the other hand, C-reactive protein (CRP) is less specific in differentiating bacteraemia and postoperative inflammatory response and has slower dynamics than PCT, with a peak serum level on the third postoperative day [8–10].
Confirmation of sepsis by BC is often inconclusive due to the long BC time-to-positivity, often more than 48 h, and because of the increasing frequency of BC negative sepsis [11–13]. The aim of this study was to examine levels of selected standard biomarkers levels in surgical septic patients and determine their predictive role in differentiating gram(+) from gram(–) sepsis.
METHODS
After approval from the local Ethics Committee (R1-14554/2022), the medical data of patients admitted to the surgical ICU of University Hospital Osijek in the period from January 2019 to May 2022 were retrospectively collected. The study included septic patients who had at least one positive BC during hospitalization. Sepsis was defined as new-onset organ dysfunction based on SOFA score [14], and in addition, the new rise in PCT with the presence of at least 2 of the following clinical criteria: fever > 38.5°C, new-onset thrombocytopaenia < 150 × 109/L without evidence of bleeding, hypo-tension requiring the use of vasopressors, new-onset rise in blood urea nitrogen, and new-onset disturbance of consciousness [15]. When sepsis was suspected, blood samples were obtained from the arterial cannula, peripheral vein, and central vein if a central venous catheter was present. In sterile conditions, 8–10 mL of blood was collected from each sampling site using BC Bactec Plus aerobic and an anaerobic medium. Patients with either only gram(+) or only gram(–) isolates were analysed, while patients with fungal and mixed isolates were excluded from further analysis. Serum levels of PCT, CRP, and white blood cells (WBC) and platelet counts were recorded on the day of BC sampling and over the following 3 days, as well as the outcome of treatment in the ICU. The study is registered at ClinicalTrials.gov under the number NCT05605275.
According to the serum PCT level in the study of Demirdal et al. [16], to observe the predictive value of serum PCT levels in distinguishing gram(+) and gram(–) sepsis, with a significance level of 0.05 and a test power of 90%, a minimum of 32 patients was required in each group (calculation made using the software G*Power, Version 3.1.9.6, Franz Faul, Universität Kiel, Germany). The normality of data distribution was tested by the Shapiro-Wilk test. Numerical data are presented as median and interquartile range, and categorical data as absolute and relative frequencies. Differences between continuous data were tested with the Mann-Whitney U test, and between categorical data with Fisher’s exact test. Binomial logistic regression was used to examine the predictive value of independent variables in differentiating the type of isolate from BC and the outcome of treatment. Receiver operating characteristic (ROC) curve analysis with a 95% confidence interval (CI) was used to define the ability of serum biomarkers to distinguish between gram(+) and gram(–) sepsis, and Youden’s indices were calculated to find the best discriminatory cut-off value. The level of significance was set at < 0.05. All P-values are 2-sided. Statistical analysis was performed using IBM SPSS software (IBM Corp. Released 2021. IBM SPSS Statistics for Macintosh, Version 28.0. Armonk, NY: IBM Corp).
RESULTS
The study included 81 septic patients from the surgical ICU who had at least one positive BC with either a gram(+) or gram(–) isolate during hospitalization. Patient characteristics, the timing of the first positive BC detection, types of surgery, and patient outcomes are presented in Table 1. The types of BC isolate are shown in Table 2.
TABLE 1
Characteristics of the patients, time of appearance of blood isolates, type of surgery, and outcome of patients
TABLE 2
The types of blood culture isolate
At the time of BC sampling, 93.8% of patients were receiving antibiotic therapy. There was no difference in the sensitivity of blood isolates to the current antibiotic treatment between patients with gram(+) and gram(–) isolates, i.e. 43.9% and 60% (P = 0.18), respectively.
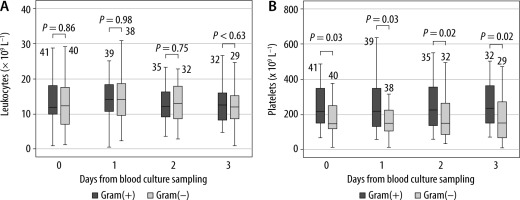
Patients with a gram(–) isolate had a significantly higher serum PCT level in all measurements compared to patients with a gram(+) isolate (Figure 1A). The serum level of CRP was significantly higher in patients with gram(–) isolates only one day after BC sampling (Figure 1B). WBC count was the same across gram(+) and gram(–) BC isolates (Figure 2A). Patients with gram(–) isolates had significantly lower platelet count in all measurements (Figure 2B).
FIGURE 1
Serum levels of procalcitonin (A) and C-reactive protein (B) regarding the type of blood culture isolate. Legend: The number of patients is written above the boxplot
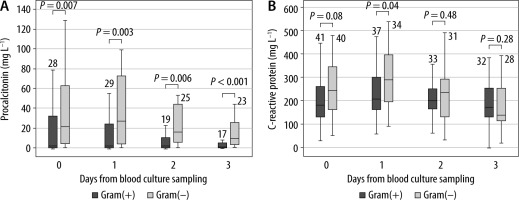
FIGURE 2
Leukocytes count (A) and platelets count (B) with regard to the type of blood culture isolate. Legend: The number of patients is written above the boxplot
The diagnostic accuracy of PCT, CRP, and platelets in distinguishing gram(–) from gram(+) BC isolates was assessed using area under the curve (AUC) analysis of ROC curves. High PCT levels and low platelet counts in all measurements were significant predictors of gram(–) isolates. The highest predictive values for PCT (Figure 3A) and platelets (Figure 3B) were observed on the third day after BC sampling, with an AUC of 0.821 (95% CI: 0.692–0.950, P = 0.001) and 0.676 (95% CI: 0.541–0.811, P = 0.02), respectively. Although platelets demonstrated significant diagnostic accuracy across all measurements, they had a lower Youden Index compared to PCT (Table 3). Furthermore, high CRP levels only day after BC sampling were also a significant predictor of gram(–) isolates, with an AUC of 0.643 (95% CI: 0.511–0.774, P = 0.04).
FIGURE 3
The ROC curve of PCT (A) and platelets (B) in distinguishing gram(–) from gram(+) blood culture isolates. PCT – procalcitonin, BC – blood culture sampling
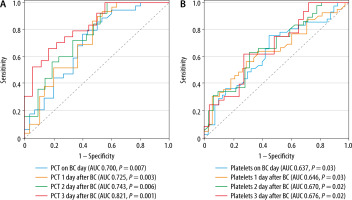
TABLE 3
The ROC curve parameters for procalcitonin (PCT) and platelets in predicting gram(–) blood culture isolates
In the multivariate logistic regression analysis assessing the likelihood of gram(–) BC isolates, with PCT, CRP, and platelet levels as predictors, it was observed that platelet counts on the day following BC sampling and PCT levels on the third day after BC sampling made a statistically significant contribution to distinguishing gram(–) from gram(+) BC isolates (Table 4).
TABLE 4
Multivariate binomial logistic regression assessing the likelihood of gram (–) blood culture isolates occurring, with procalcitonin (PCT), CRP, and platelets as independent predictors
There were no significant differences in serum PCT, WBC, and platelet counts between survivors and non-survivors. However, the median CRP levels 2 and 3 days after BC sampling were significantly lower in survivors, with values of 183.8 (114.9–268.5) vs. 239.4 (189.8–305.9) mg L–1 (P = 0.03) and 144 (104.8–186.1) vs. 223.1 (130–287.7) mg L–1 (P = 0.03), respectively. Multivariate regression model with age, PCT, CRP, and platelet count for each day as an independent variable showed that age and serum CRP levels were significant predictors of death outcome (Table 5).
TABLE 5
Multivariate binomial logistic regression assessing the likelihood of death outcome, with age, procalcitonin (PCT), C-reactive protein (CRP), and platelets as independent predictors
DISCUSSION
The study’s findings indicate that serum PCT and platelet counts can assist in distinguishing between gram(+) and gram(–) sepsis in surgical patients. Previous research has confirmed a stronger induction of extrathyroidal PCT synthesis by gram(–) bacteria, but there is a lack of studies focusing on critically ill surgical patients. The exact mechanism of stronger induction of PCT synthesis by gram(–) bacteria has not been fully elucidated. A possible cause is a difference in the membrane structure of gram(+) and gram(–) bacteria [17–22]. In a study conducted by Leli et al. [23], the median PCT level was 13.8 μg L–1 in gram(–) sepsis and 2.1 μg L–1 in gram(+) sepsis. A cut-off value of 10.8 μg L–1 demonstrated 60% sensitivity and 82% specificity in discriminating between gram(–) and gram(+) isolates. However, it is important to note that only 9.2% of patients in this study were surgical patients. In another study by Li et al. [24], which involved 328 episodes of bacteraemia, the median PCT level in gram(–) sepsis was 7.47 μg L–1, and the optimal PCT cut-off value for distinguishing between gram(–) and gram(+) pathogens was 2.44 μg L–1. Unfortunately, the specific type of patients included in this study was not described.
Our research findings showed that the median PCT level in gram(–) sepsis was twice as high as reported in the study by Leli et al. [23]. This difference may be attributed to the synergistic induction of PCT synthesis by surgery and postoperative sepsis, especially since the mentioned study included less than 10% surgical patients. Notably, Barbić et al. [25] demonstrated that uncomplicated abdominal surgery led to an increase in PCT levels on the first postoperative day, reaching up to 1.17 μg L–1. Subsequently, PCT concentrations exhibited a declining trend in the following days, but the median concentration remained elevated compared to the baseline preoperative value, even in the absence of bacterial infection. The decrease in PCT levels observed in our patients on the second day after blood culture sampling can probably be attributed to the timely administration of broad-spectrum antibiotics.
The findings from our study highlight the predictive capability of PCT across all measurements in distinguishing between gram(+) and gram(–) BC isolates. However, the highest predictive value for PCT in distinguishing gram(–) from gram(+) pathogens was achieved 3 days after BC sampling. Even this delayed prediction can hold clinical significance, particularly given the rising occurrence of BC-negative sepsis, where identifying the causative agent is challenging [13].
Low platelet levels are common in ICU septic patients. Coagulation dysfunction in sepsis is primarily a reflection of endothelial damage and activation of platelets by pro-inflammatory mediators [26, 27]. Non-resolution of thrombocytopaenia is associated with a higher risk of bleeding, increased organ dysfunction, and higher mortality in septic patients [28, 29]. The aetiology of thrombocytopaenia in septic patients is multifactorial and includes increased platelet activation and consumption during thrombus formation, reduced platelet production, and heightened platelet destruction [30]. Our study’s results demonstrate that thrombocytopaenia is more pronounced in patients with gram(–) sepsis, which is probably a consequence of a stronger immune response that causes stronger endothelial dysfunction. Wagner et al. [31] proved using culture of human endothelial cells that TNF-a and PCT cause endothelial barrier disruption and impair endothelial cell function. Consequently, elevated PCT levels may contribute to heightened platelet exposure to subendothelial adhesive molecules, increased platelet aggregation, and the formation of microthrombi.
Unlike PCT, CRP is a nonspecific biomarker of inflammation. Synthesis of CRP and an increase in its serum level are correlated with pro-inflammatory cytokines [32–34]. In surgical patients, elevated CRP levels have been observed in various conditions such as wound infections, anastomosis leakage, pneumonia, urinary tract infections, the development of a postoperative systemic inflammatory response, postoperative bleeding, and numerous other conditions associated with elevated proinflammatory cytokine levels, which may not necessarily be related to bacteraemia and sepsis [10, 35–38]. However, the role of serum CRP level in differentiating infectious and non-infectious postoperative complications has been confirmed by numerous studies. In a study by Plat et al. [35] on elective surgery patients, CRP levels reached 292 mg L–1 in patients with infectious complications and 173 mg L–1 in those without on the second postoperative day. High CRP levels in surgical septic patients reflect a combination of nonspecific systemic inflammation and bacterial infection. While CRP synthesis can be induced by various conditions, its utility in distinguishing between gram(–) and gram(+) sepsis remains uncertain. However, high levels of CRP were associated with a worse outcome. This result is consistent with the results of other studies that have demonstrated that high levels of CRP in critically ill patients are associated with postoperative complications, organ dysfunction, and higher mortality [37, 39, 40].
The major limitation of this study is its retrospective nature. Retrospectively defining sepsis in the ICU is challenging due to its varied presentations, overlap with other conditions, dynamic characteristics, subjective elements, subtle early signs, and evolving diagnostic criteria [15, 41]. Additio-nally, important patient demographic factors that could influence CRP and PCT levels, such as chronic heart, liver, and kidney diseases, were not considered in this analysis [42–44]. Furthermore, this study did not investigate the source of other infections, such as peritonitis or pneumonia. The study’s focus exclusively on septic patients with positive blood cultures and either gram(+) or gram(–) sepsis may introduce selection bias by excluding patients with negative cultures or other types of infections, potentially limiting the applicability of the results to a broader septic population. Conducting a new prospective study that addresses these limitations could provide more precise insights into the dynamics of CRP and PCT in surgical septic patients.
CONCLUSIONS
High PCT levels in surgical septic patients show potential in distinguishing between gram(–) and gram(+) sepsis, especially when thrombocytopaenia is also present. However, further study with a larger and more homogeneous sample of surgical patients is essential to better understand the specific role of serum biomarkers in identifying the causative agent of sepsis.




