Introduction
Sepsis is a serious, complex disease characterized by a systemic inflammatory response triggered by severe infection of the organism, which can lead to various life-threatening tissue damage and organ dysfunction, especially the kidney, an extremely susceptible organ [1-3]. Acute kidney injury (AKI), characterized by a dramatic decline in renal function, is one of the most common and serious complications of sepsis. Up to 50% of patients with AKI experience sepsis, and the proportion of sepsis patients developing AKI in the late stage reaches 60% [4-6]. The latest consensus, published in 2023, of the 28th Acute Disease Quality Initiative (ADQI) makes it clear that sepsis accounts for 45-70% of all cases of AKI among critically ill patients [7], which is closely associated with high mortality. These data indicate that sepsis-associated AKI is an unignorable threat to the healthy survival of all mankind.
Although emerging research on the epidemiology, pathophysiology, and clinical risk factors of sepsis-associated AKI has advanced diagnosis and treatment, there is still a significant lack of specific treatment methods in clinical practice. Currently, some indicators are applied for early evaluation of kidney injury, such as serum creatinine (Scr), cystatin-C (Cys-C), neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), tissue inhibitor of metalloproteinase-2 (TIMP-2), and insulin-like growth factor binding protein (IGFBP7). However, their limited diagnostic performance cannot satisfy the clinical demands of most of them [8]. Moreover, the mechanism of sepsis-associated AKI remains to be elucidated [4]. Existing studies have found that many factors are related to the occurrence and development of AKI, including inflammation, intracellular endotoxin, mitochondrial dysfunction, and microcirculation disorder, among others [9]. In recent years, many studies have shown that the inflammatory response is an important pathogenesis of AKI [10-13]. Similarly, the 28th ADQI consensus points out that sepsis-associated AKI is also closely related to abnormal inflammation, in addition to microcirculation disorders and cellular metabolic reprogramming. Thus, it can be seen that it is of great significance to explore new biomarkers with high specificity and sensitivity for the early prediction and diagnosis of AKI in patients with sepsis.
MicroRNAs (miRNAs) are short-sequence RNAs derived from genomic DNA with regulatory functions that are widely present in eukaryotes and do not have an open reading frame. Previous studies have shown that miRNAs are implicated in various diseases by regulating the expression of target messenger RNA [14-16]. In addition, some studies have found that changes in miRNA expression levels may contribute to the treatment of AKI [17-21]. For instance, in HK2 cells treated with lipopolysaccharide (LPS), the expression of miR-20a and miR-942-5p was down-regulated while the corresponding inflammatory factors were up-regulated [22, 23]. In a rat model of sepsis established by CLP (cecal ligation and puncture) surgery, increasing the expression of miR-22-3p and miR-191-5p reduces the inflammatory response [24, 25]. Reduced inflammation alleviates LPS-induced kidney injury [26]. Previous studies have found that miR-582-5p is down-regulated in many inflammation-related diseases and plays an inflammation-inhibiting role [27-29]. Downregulation of miR-582-5p in diabetic CKD (chronic kidney disease) was also demonstrated [30]. The potent anti-inflammatory activity of miR-582-5p led us to speculate that miR-582-5p might be a potential candidate for predicting and evaluating sepsis-induced AKI.
In this study, sepsis-induced AKI patients were employed to validate the predictive value of miR-582-5p for AKI occurrence, which could provide a reference for screening novel biomarkers of AKI prediction, diagnosis, and even treatment for clinical application. In addition, an LPS-induced HK2 cell model was established to examine the functional role of miR-582-5p in AKI.
Material and methods
Clinical patients and sample collection
One hundred and eighty patients with sepsis were continuously enrolled in this study at Chengdu BOE Hospital. The basic clinical characteristics of these informed and consented patients were analyzed as presented in Table 1. All diagnoses of sepsis and AKI were made according to the clinical criteria, and hospitalized patients were divided into two groups. The non-AKI group included patients whose condition did not progress to AKI, while the AKI group included those who developed AKI subsequently, during sepsis. Recently, the definition of sepsis-induced AKI needed to meet the following requirements simultaneously: Firstly, patients should be at least than 18 years old. Then, within 48 hours, the Scr increase should be at least 0.3 mg/dl, or the increase within 1 week should be at least 1.5 times the baseline. In addition, the exclusion criteria are as follows: Pregnant women, patients after kidney transplant surgery, and those who suffered nephrotoxin within 4 weeks prior to hospitalization were not considered. Moreover, patients with conditions such as severe hepatitis, immunodeficiency, other chronic kidney disease, or obstructive uropathy were also excluded. This study was conducted with the permission of the Ethics Committee (no. 20220106) and with informed consent obtained from each patient. After hospitalization, peripheral blood and urine samples were collected in a timely manner with the patient’s permission, which was prior to any treatment. Blood samples for clinical hematology detection were collected into anticoagulant blood collection vessels with EDTA-K2 and blood samples for serum were collected without anticoagulant. The serum and urine samples were obtained by centrifugation at 3000 rpm for 10 minutes and 5 minutes, respectively. Prepared samples were stored at –80°C promptly for later examination.
Table 1
Clinical characteristics of the study groups
[i] BMI – body mass index, APACHE – Acute Physiology and Chronic Health Evaluation, SOFA – Sepsis-related Organ Failure Assessment, CRP – C-reaction protein, PCT – procalcitonin, WBC – white blood cells, eGFR – estimated glomerular filtration, Scr – serum creatinine, Cys-C – cystatin-C, NGAL – neutrophil gelatinase-associated lipocalin, KIM-1 – kidney injury molecule-1
Cell culture and transfection
Human HK-2 cells purchased from the Cell Bank of the Chinese Academy of Sciences were cultured in RPMI-1640 medium (Gibco, 11875093) with 10% FBS (Gibco, 26010074), following routine aseptic culture procedures. To establish a valid model, different durations of exposure to LPS (Solarbio, IL2020) were necessary for HK-2 cells, referring to existing studies. Furthermore, HK-2 cells were treated with 10 µg/ml LPS for 12 hours for the simulation of AKI in vitro [23, 31]. Before transfection, LPS-induced cells were transferred to 24-well plates at the density of 5 × 104/well. An approximate confluence of 80% was favorable for cell transfection. Exogenous RNA was introduced into the cells by the Lipofectamine 3000 (Thermo Fisher Scientific, L3000001) reagent. The miR-582-5p mimic (miR-mimic, 5'-UUACAGUUGUUCA ACCAGUUACU-3') and negative control (mimic-NC, 5'-3’GGACCAAATCTCGAGATTTGG) were specially synthesized by Genepharma in China. The elevated miR-582-5p level was regulated by the corresponding miR-mimic in the following scenario. Cell transfection consisted of 4 groups, including the control group, LPS group, LPS + mimic-NC, and LPS + miR-mimic.
qRT-PCR
RNA extraction with Trizol reagent (Invitrogen, 12183555CN) was carried out according to the specification. For tissue samples, it was necessary to homogenize with nuclease-free grinding rods (Sangon, F619071) after adding Trizol reagent, and vortexing was sufficient for cell samples. The complementary DNA synthesized by the conventional reverse transcription kit (Qiagen, 218061) was used for the qRT-PCR test with the kit (Takara, RR420A). The discrepant gene expression was evaluated with β-actin as an internal control according to the 2-ΔΔCt methods. The brief amplification conditions were predenaturation at 95oC for 30 s followed by denaturation at 95oC for 30 s, annealing at 60oC for 40 s, and extension at 72oC for 30 s, which were performed in 40 cycles.
Cell viability assay
Cell viability after LPS treatment and/or diverse transfections was estimated by the CCK-8 Kit (Solarbio, CA1210) based on the standard curve prepared according to the specifications. 96-well plates were used for cell seeding, with 1 × 104 cells per well. HK-2 cells were exposed in 10 µg/ml LPS for 0 hours, 6 hours, 12 hours, and 24 hours. These cells exposed for different times were duly collected for subsequent assays. The cells without any treatment were regarded as the negative control. Cell Counting Kit-8 (CCK-8) reagents (10 µl/well) were added and any air bubble were avoided. Then they were incubated for two hours under normal cell culture conditions. An enzyme immunoassay analyzer was necessary to detect the optical density value in each well at 450 nm.
ELISA analyses
The kidney injury assessment as well as inflammatory response detection were conducted by means of ELISA. Both main renal injury indicators and inflammatory factors were detected with an ELISA kit. An automatic biochemical analyzer was applied for content determination of cystatin-C (Elabscience, E-EL-H3643) and Scr (Njjcbio, C011-2-1) to assess kidney injury, and additional measurements of NGAL (Elabscience, E-EL-H6127) and KIM-1 (Elabscience, E-EL-H6029) were also conducted. On the other hand, cytokines including interleukin (IL)-1β (Elabscience, E-EL-H0149) and IL-6 (Elabscience, E-EL-H6156), along with tumor necrosis factor α (TNF-α; Elabscience, E-EL-H0109), were also used to indicate the inflammation level. The concentration was calculated based on the standard curve obtained from a standard sample on the same ELISA plate. It should be noted that both serum samples and cell supernatants needed centrifugation at 4oC to remove impurities, strictly following the instructions of the ELISA kit. Additionally, the blank control was also necessary for the ELISA assay.
Cell apoptosis test
The Annexin V-FITC/PI Double Staining Cell Apoptosis Assay Kit (Beyotime, C1062S) was used to detect apoptotic cells. HK-2 cells after treatment were trypsinized and collected by centrifugation. After washing three times with 1 × phosphate-buffered saline (PBS) buffer, which was important for subsequent experiments, cell pellets were resuspended in binding buffer. Propidium iodide and Annexin V-FITC dye were added to the cell suspension. Then, it was necessary to incubate for 15 minutes in the dark at room temperature. Slight shaking during the incubation process was beneficial to improve staining. The cells must be examined as soon as possible after staining, usually within an hour. Finally, a flow cytometer was used to assess cell apoptosis.
Dual-luciferase reporter assay
The wild-type 3'UTR of HMGB2 (WT-HKGB2) and mutant (MUT-HMGB2) were severally cloned into the pGL3 vector (Promega, E1741). Subsequently, recombined vectors were co-transfected with miR-582-5p-mimic and miR-582-5p-mimic-NC separately into HK-2 cells. The activation performed by luciferase was detected in the dual-luciferase reporter system kit (Promega, E1960) 48 h after transfection.
Oxidative stress assay
In LPS-induced HK-2 cells, the oxidative stress level was identified by classical indicators including reactive oxygen species (ROS), malondialdehyde (MDA), and superoxide dismutase (SOD). These metrics were quantized by the corresponding assay kit (Beyotime, S0033M, S0131M, S0101M), according to the manufacturer’s protocols and multifunctional microplate reader. Cells were harvested by centrifugation at 600 g for 5 min and washed once with PBS pre-cooled at 4oC or by an ice bath. The cells were properly resuspended after adding an appropriate amount of sample preparation solution to fully lyse. Then, samples were then centrifuged at 12,000 g at 4oC for 3-5 min, and the supernatant was taken as the sample to be tested. During the experiment, blank control groups were necessary.
Statistical analysis
All data were analyzed using SPSS Statistics 23.0 or GraphPad Prism 7.0 and they were presented as mean ±SD. Differences of continuous variables between two groups were calculated by unpaired Student’s t-test, and the one-way ANOVA test was applied for multi-group comparison. The chi-square test was used for comparison of categorical variables. The diagnostic performance was calculated using the receiver operator characteristic (ROC) curve. Pearson’s correlation analysis was applied for correlation examination. Statistical significance was set at a p value less than 0.05.
Results
Baseline characteristics of sepsis patients
Clinical information of all admitted sepsis patients was recorded and exhibited in Table 1. Comparative analysis of multiple indicators indicated no difference between non-AKI and AKI groups, such as BMI, common complication, age, in addition to gender. Also, Sepsis-related Organ Failure Assessment (SOFA) levels in AKI patients were not significantly different to those in non-AKI patients. Levels of Scr, NGAL, eGFR, CRP, PCT, and WBC were higher in AKI patients (p < 0.05). By contrast, levels of APACHE II, Cys-C, and KIM-1 were significantly lower in non-AKI patients (p < 0.001).
Down-regulated expression of miR-582-5p in sepsis-induced AKI patients
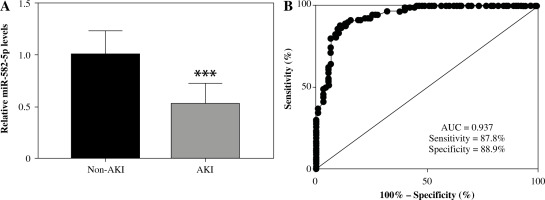
To investigate the role of miR-582-5p in sepsis-induced AKI, samples from 180 patients were collected for RNA extraction and subsequent qRT-PCR through conventional experimental procedures. As shown in Figure 1A, the expression levels of miR-582-5p in the non-AKI group were significantly higher than those in the AKI group (p < 0.001). This suggested that miR-582-5p may be associated with sepsis-induced AKI. Correlation analysis also showed the significant correlation between serum miR-582-5p and markers of kidney injury including Scr, Cys-C, NGAL, and KIM-1 (Table 2).
Fig. 1
Expression of miR-582-5p in sepsis patients and its diagnostic performance. A) Relative expression of miR-582-5p in sepsis-induced acute kidney injury (AKI) patients (n = 90), compared to the non-AKI groups (n = 90). B) Evaluation of the diagnostic ability of miR-582-5p. Each sample was bio-replicated 3 times. A) Student’s t test. ***p < 0.001 compared to non-AKI group
Diagnostic performance of miR-582-5p for early screening of AKI from sepsis patients
Further investigations focused on the diagnostic performance of miR-582-5p. The ROC curve was applied to confirm previous speculation. As shown in Figure 1B, the area under the miR-582-5p ROC curve was 0.937, which displayed a relatively satisfactory sensitivity and specificity of 87.8% and 88.9%, respectively. Cutoff values were determined based on the maximum of Youden’s index to calculate the optimum sensitivity and specificity.
Up-regulated miR-582-5p improved cell viability and inhibited apoptosis
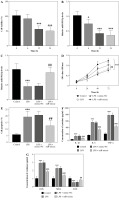
In LPS-induced HK2 cells, we found that with the passage of time, a significant downward trend was observed in cell viability (Fig. 2A) and the relative level of miR-582-5p (Fig. 2B). In order to further explore the role miR-582-5p played, cell transfection was employed to enhance its expression level (Fig. 2C). The up-regulated miR-582-5p significantly reversed the declining cell viability measured at 72 h after transfection (Fig. 2D), along with high apoptosis (Fig. 2E).
Fig. 2
Function of miR-582-5p in LPS-induced HK2 cells. A) Cell viability decreased with time. B) Relative expression of miR-582-5p decreased with time, assessed by CCK-8 kit. C) Successful cell transfection verified by qRT-PCR of miR-582-5p. D) Poor cell viability was improved by increased miR-582-5p. E) The proportion of cell apoptosis decreased with increased miR-582-5p. F) Up-regulated miR-582-5p diminished the inflammatory response. Three repetitions were required for each experiment and each sample was bio-replicated 3 times. A-G) Student’s t test. *p < 0.05 compared to control; ***p < 0.001 vs. control group; ##p < 0.01 vs. LPS + mimic-NC; ###p < 0.001 vs. LPS + mimic-NC. G) Highly expressed miR-582-5p enhanced the ability to resist oxidative stress. Three repetitions were required for each experiment and each sample was bio-replicated 3 times. G) Student’s t test. *p < 0.05 compared to control; ***p < 0.001 vs. control group; ##p < 0.01 vs. LPS + mimic-NC; ###p < 0.001 vs. LPS + mimic-NC
Enhanced miR-582-5p suppressed inflammatory response and oxidative stress
Considering that inflammation plays an important role in the development of AKI, we focused on whether the highly expressed miR-582-5p affected the inflammatory response. In HK-2 cells treated with LPS, the concentrations of typical cellular inflammatory factors such as IL-1β, IL-6, and TNF-α were significantly increased. However, unexpectedly, elevated miR-582-5p expression significantly attenuated the high inflammatory trend (Fig. 2F). A similar function was also found in oxidative stress. Compared with the control group, increasing expression of miR-582-5p decreased the concentrations of ROS and MAD and increased the concentration of SOD at the same time (Fig. 2G). Briefly, up-regulated miR-582-5p contributed to inhibiting inflammation and accelerating antioxidants.
HMGB2 could be regulated by miR-582-5p by direct targeting
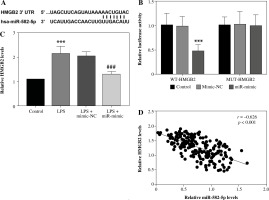
The online database ENCORI was applied to screen interacting target genes. HMGB2 was found to have complementary paired sequences with miR-582-5p (Fig. 3A). Based on this, a dual-luciferase reporter assay was performed for further analysis. After transfection with miR-582-5p mimics, luciferase activity was distinctly weakened for WT-HMGB2. By contrast, the enhancement of miR-582-5p had no effect on luciferase activity for MUT-HMGB2 (Fig. 3B). Hence, miR-582-5p targeted HMGB2 directly in HK-2 cells. The measurement of the relative level indicated that increased HMGB2 expression was diminished by raised miR-582-5p (Fig. 3C). The correlation analysis indicated that serum HMGB2 levels were negatively correlated with miR-582-5p levels (r = –0.626, Fig. 3D).
Fig. 3
MiR-582-5p regulated HMGB2 in a targeted manner. A) Complementary binding sites between miR-582-5p and HMGB2. B) Dual-luciferase reporter assay to analyze the interaction of miR-582-5p and HMGB2 in HK-2 cells. C) Expression level of HMGB2 measured by qRT-PCR. D) Correlation between miR-582-5p and HMGB2 by Pearson correlation analysis. Three repetitions were required for each experiment and each sample was bio-replicated 3 times. B, C) One-way analysis of variance. Student’s t test. ***p < 0.001 compared to control group, ###p < 0.001 compared to LPS + mimic-NC
Discussion
Sepsis-induced AKI is a complex, universal disease with high incidence and mortality, especially for critical patients [32]. Its unknown and puzzling pathophysiology may account for the lack of effective means in prevention and treatment [33]. Here, we found evidence of the reliable predictive value of miR-582-5p for sepsis-induced AKI patients.
Non-coding RNAs participate in many physiological processes in an organism by affecting the expression of genes [34, 35]. Functional studies of miRNAs have shown that they are closely implicated in a variety of diseases [36, 37]. When disease occurs, miRNA in diseased tissues or organs will present specific expression profiles [38, 39]. The expression dysregulation may act as a tumor suppressor or oncogene [40, 41]. In this study, it was found that relative expression of miR-582-5p was apparently lower in both sepsis-induced AKI patients and LPS-induced HK2 cells. In an in vitro cell model, HK2 cells exhibited reduced cell viability and increased apoptosis. However, high expression of miR-582-5p by transfection improved cell viability and apoptosis to a large extent. A similar situation has been documented in previous cancer-related studies [42-44]. For instance, in colorectal cancer patients, miR-582-5p was proven to be lowly expressed, which enhanced the migration of tumor cells [45]. High miR-582-5p expression greatly increased cell viability and simultaneously reduced apoptosis through ROCK1 [46]. It was also found that in CRC (colorectal carcinoma) tissues, miR-582-5p showed lower expression, and it plays an important role in cell cycle arrest and apoptosis [47]. The above results also indicated that miR-582-5p had predictive value.
Inflammation and oxidative stress are inseparable from the disease process. Oxidative stress originates from the imbalance between the production and elimination of oxygen-free radicals in the body or cells, which is widely regarded as an important factor accelerating many diseases and aging [48]. Continuous oxidative stress can induce an inflammatory response, which can mediate a variety of diseases [49]. Various transcription factors can also be activated by oxidative stress, such as PPAR-γ, Nrf2, and P53, and thereby induce expression of multiple genes, including cell cycle regulatory factors and inflammatory factors [50, 51]. In our research inflammatory factors and oxidation levels showed apparent mitigation following cell transfection of miR-582-5p mimics, which was consistent with previous reports. Increasingly, studies have indicated that miR-582-5p reduces inflammatory-associated factors including TNF-α and IL-1β, in various mice models and cell models [27, 52, 53]. It has also been reported that by regulating inflammation, along with oxidative stress, miR-582-5p overexpression is conducive to amelioration of PC12 cell injury [28].
HMGB2 is a chromatin-associated protein that plays a role in transcription, cell proliferation and tumorigenesis [54]. Further analysis in this research identified HMGB2 as a potential target for miR-582-5p. The expression of HMGB2 was elevated in LPS-induced HK2 cells. However, it decreased following an increase of miR-582-5p expression, revealing a negative correlation between them. In previous studies, HMGB2 has been implicated in inflammation and oxidative stress. HMGB2 is involved in the regulation of abdominal aortic aneurysm inflammation through NF-κβ signaling [55]. In osteoarthritis, HMGB2 interacts with circSLTM and participates in the regulation of inflammation and apoptosis [56]. HMGB2 is also proven to target miR-223 in acute lung injury models exposed to LPS and mediates oxidative stress and autophagy [57, 58].
In brief, downregulated miR-582-5p serves as a biomarker for sepsis-induced AKI. Elevated miR-582-5p expression was beneficial to HK-2 cell viability through mediating oxidation and inflammation. Although our results showed that miR-582-5p played an important role in regulating sepsis-induced AKI and miR-582-5p functioned perhaps through HMGB2, due to some differences between the cell model and the actual clinical application, the role and regulatory mechanism of miR-582-5p in sepsis-induced AKI still require further confirmation in vivo.


