Introduction
Hepatitis delta virus (HDV) is a virus that has ribonucleic acid (RNA) for its genetic material and was first detected in 1977 in Italy. It is the smallest known virus that is capable of infecting mammals. Although HDV causes the most aggressive and rapidly progressive form of viral hepatitis, current literature still lacks comprehensive data about its prevalence. There is also limited awareness of the risk factors and dangers of HDV infection in the general population. Moreover, many research centers and laboratories do not conduct RNA testing crucial for diagnosing active infections.
The route of infection is parenteral [1]. The virus spreads only among individuals previously infected with hepatitis B virus (HBV). This is because HDV has no envelope of its own and needs HBV surface antigen (HBsAg) to build its outer coat [2]. However, studies indicate that HDV spread is also possible through mitosis in an HBV-independent way, but this process needs further study. Simultaneous infection with both HBV and HDV is called co-infection, whereas HDV infection in a patient with chronic hepatitis B is referred to as superinfection. Both co-infection and superinfection may lead to exacerbation of liver disease due to the damaging effects of HDV on the hepatic tissue already infected by HBV [3].
Despite the need for accurate epidemiological data, these are still insufficient. It is estimated that there are 12 million people worldwide already infected [4]. The actual number may be different as the global HDV prevalence is difficult to determine due to the heterogenic character of disease geographic distribution. The prevalence of hepatitis B and hepatitis D together is estimated at 4.5% (1 in 22 HBsAg-positive people). The presence of HDV infection among HBV-infected individuals is especially common in Central and West Africa as well as Central and North Asia. In Europe, the highest presence of anti-HDV antibodies among HBV-infected patients was reported for Romania (23%), eastern Turkey, Russia and Greenland, while the world leader is Mongolia (36.9% co-infections) [4, 5]. To date, epidemiological data on the prevalence of anti-HDV antibodies in the Polish population are unsatisfactory and based on a few studies with an estimated seroprevalence of 5% [6, 7]. Moreover, current data do not show the frequency of HDV infection in the Silesian population. Detailed information on existing data in the Polish population are presented later in this article.
As HDV can cause both acute and chronic hepatitis it is considered a highly pathogenic virus. Hepatitis D may progress towards cirrhosis and liver decompensation. HBV-HDV superinfected patients are at higher risk of developing severe hepatitis due to the spread of HDV and aggravation of pre-existing liver illness. Frequently, the superinfection may advance to acute liver failure requiring an organ transplant [8]. The potential role of HDV in the progression to hepatocellular carcinoma (HCC) is also taken into consideration [9]. The result of late disease diagnosis is therefore rapid progression and the most severe form of hepatitis.
In response to the need to further investigate the prevalence of HDV infection, we present results from our study on the Polish population. The main goal of this research paper is to establish the frequency of HDV infection among HBV infected patients. Secondly, we collected data about risk factors and course of the disease among infected patients and compared our data with current literature.
Material and methods
This retrospective study was conducted during the period between 03.2020 and 11.2022 in a single hepatologic center of specialized care (ID Clinic, Myslowice, Silesia, Poland). 177 patients with confirmed HBV infection were examined for HDV superinfection. Importantly, in some of the patients HDV infection was already suspected, with the first diagnosis of HDV infection made in the period 2015-2020.
The diagnostic methods used in this study included the assessment of HDV total antibodies, immunoglobulin (Ig) M antibodies and HDV antigen by the commercially available set DIA.PRO (Milano, Italy) according to manufacturer recommendations. HDV total antibody levels were detected with the third generation Enzyme ImmunoAssay (ELISA) DiaPro HDV Ab (CE 0318) kit [10], for IgM antibody count the ELISA kit DiaPro HDV IgM (CE 0318) was used [11] and HDV antigen levels were determined using the ELISA DiaPro HDV Antigen set all with sensitivity and specificity both > 98% [12]. HDV RNA testing was not conducted, due to the retrospective character of the study. Alanine aminotransferase (ALT) was measured, with reference values of < 40 U/l. Patients with hepatitis D also had a test analyzing the liver fibrosis stage – mostly the FibroScan and some of them also the liver biopsy. The interpretation of the results was according to the METAVIR score with the threshold suggested by the manufacturer (F0 means no scarring, F1 is mild fibrosis, F2 is moderate fibrosis, F3 is severe fibrosis and F4 is cirrhosis or advanced fibrosis). A telephone follow-up of patients who tested positive indicating HDV infection was also conducted, in which they were asked about their current health status, the course of HDV infection and possible risk factors.
Results
Of the 177 patients diagnosed for HBV infection, the majority (n = 117, 66%) were male. A positive test for HDV total antibodies was obtained in six patients, indicating the infection with hepatitis D virus. This estimates a prevalence of HDV infection in the Silesia region of 3.4%.
Among hepatitis D patients, there were more men (n = 4) and all patients were middle-aged. Four patients already had an advanced stage of fibrosis (F3 or higher) before starting treatment, one of them having undergone liver transplantation. ALT levels in HDV patients were above normal in half of the cases (Table 1). Some patients had other comorbidities. Half of the hepatitis D cases were observed to have risk factors for possible sexual transmission, blood transfusions, or intravenous drug use.
Table 1
Clinical characteristics of infected patients: demographics, virological and fibrosis assessment
| ID | Gender | Age (years) | Detection of HBV infection | HBV DNA before treatment (U/ml) | HBV DNA after treatment (U/ml) | Fibrosis stage by FibroScana before treatment | Fibrosis stage by FibroScana after treatment | Detection of HDV infection | ALT (U/l)c |
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | F | 56 | 2009 | 3218 | Ub | F3 | F1 | 2016 | 22 |
| Patient 2 | F | 55 | 2017 | 2120 | U | F2 | F0/F1 | 2019 | 18 |
| Patient 3 | M | 70 | 2005 | 250 | < 10 | F4 | F4 | 2017 | 322 |
| Patient 4 | M | 64 | 1999 | 15 | U | F4 | F1 (liver transplantation) | 2015 | 35 |
| Patient 5 | M | 45 | 2015 | 19192 | U | F1 | N/A | 2020 | 35 |
| Patient 6* | M | 55 | 2012 | N/A | U | F4 | F4 | 2020 | 47 |
Patient 1
The first case concerns a 56-year-old woman whose HBV infection was detected during pregnancy in 2009. Her viral status was: HBeAg negative, anti-HBe positive, HBsAg – 20163 U/ml. Antiviral therapy with lamivudine was initiated. Entecavir and tenofovir were then used. The patient is currently treated only with tenofovir (245 mg per day). Baseline HBV DNA was 3218 U/ml, currently undetectable (March 2023). The HBsAg level decreased to 4485 U/ml. The ALT level was 146 U/l in May 2009, while currently the level is 22 U/l. The fibrosis stage before treatment was assessed to be F3 (severe liver scarring), and after the treatment F1 (no liver scarring or mild liver scarring). Ultrasound examination of the liver showed no abnormalities (2022). High aminotransferase activity despite antiviral treatment was the reason for the decision to investigate for HDV infection, which was confirmed in 2016. The patient underwent an appendectomy at the age of 16 and has no other concomitant diseases. She denies risky behavior and visits countries with an increased risk of infection with hepatitis viruses.
Patient 2
The second HDV-positive patient is a 55-year-old woman suffering from depression. Hepatitis B was detected in 2017; at the same time she suffered from acute hepatitis A. HBeAg was then negative and the patient was anti-HBe positive. Therapy with entecavir (1 mg per day) was introduced, lasting until now. Prior to the treatment, HBV DNA was 2120 U/ml, currently undetectable. Baseline HBsAg was 4418 U/ml, presently 2197 U/ml. In-depth diagnostics due to the persistently elevated activity of liver enzymes was implemented in 2019 – HDV co-infection was detected during these tests. A FibroScan test was performed on the patient in 2022, indicating fibrosis stage F0/F1. Currently ALT levels are within the normal range (March 2023). The patient has not stayed in countries with an increased risk of HBV infection, and she denies risky behavior – only one of her sexual partners occasionally used drugs.
Patient 3
The third HDV-positive patient is a male, born in 1954, who was diagnosed with HBV infection in 2005, whereas HDV coinfection was detected in 2017. The circumstances of both infections are unknown, and the patient denies any risky behaviors.
HBV infection was diagnosed as a result of further testing after detecting abnormalities during routine examination. The disease was identified in the advanced stage with the first signs of liver cirrhosis and thrombocytopenia, but without splenomegaly.
Antiviral treatment was implemented for the patient in 2016. Initially, the therapy was conducted with pegylated interferon (PEG-IFN), but due to decompensation during treatment, that drug was substituted with the nucleotide analog tenofovir. Antiviral therapy had been conducted for 2.5 years. In 2016 the patient was diagnosed with focal lesions in two liver segments and in 2017 was eligible for a liver transplant due to suspected hepatocellular carcinoma. Once the diagnosis was ruled out, the transplant was abandoned, but HDV infection was detected at the time of evaluation for transplant.
At baseline the test for HBeAg was negative, and HBe antibodies were positive. The level of HBV DNA was 250 U/ml and the level of HBsAg was 16024 U/ml. Also, the level of HDV RNA was recorded in this patient (39,000,000 geq/ml). The patient presented liver cirrhosis (F4) and ALT activity was increased. At that time, no other concomitant diseases were reported.
After the treatment, HBV DNA was still detectable at a low level (< 10 U/ml), and the HBsAg level decreased to 6748 U/ml. In a FibroScan performed in 2022, the fibrosis stage remained at F4. ALT activity is currently (March 2023) raised to the level of 322 U/l. Due to liver cirrhosis, the patient is under constant observation by the transplant center.
Patient 4
The next patient, with both hepatitis B and D, is a 64-year-old man with hypertension and haematological abnormalities (chronic leukopenia with granulocytopenia and minor thrombocytopenia), who was diagnosed with hepatitis B in 1999 during routine examinations. At that time, despite suspicion of cirrhosis, liver biopsy was abandoned due to thrombocytopenia. The infection was probably vertical (the mother and brother were also infected). The patient was hospitalized as a child (3 or 4 years old) but does not recall the reason. Until the diagnosis was made, he had not been in countries with high risk of infection. Initially the HBV DNA level was 15 U/ml and the quantitative measurement of HBsAg was 1708 U/ml. HDV infection was detected in 2015. The fibrosis stage was then defined as F4 – cirrhosis, which led to liver transplantation in 2016 due to a long-standing HBV infection. The patient was treated with antiviral therapy for four years. Entecavir was used as the first therapy, and after the diagnosis of HDV infection, PEG-IFN was used as the second therapy. Lamivudine was used as the third therapy implemented after liver transplantation. The patient is also taking prednisone due to the hematological abnormalities. The subject achieved an undetectable HBV DNA level and HBsAg level. ALT is within the normal range (recent result – ALT 35 U/l). A FibroScan test performed on the transplanted liver showed fibrosis stage F1.
Patient 5
The case concerns a 45-year-old HDV-positive man who was diagnosed with HBV infection in 2015. The most likely source of infection was a blood transfusion after a traffic accident in 1994. Primarily the level of HBV DNA was 19192 U/ml and the level of HBsAg was 13188 U/ml. HBeAg was negative and the HBe antibodies were positive. Liver enzymes were not elevated. Before the beginning of the treatment, the fibrosis stage was graded F1, equivalent to portal fibrosis without septa. The patient received the following treatment: first PEG-IFN and later tenofovir. The therapy lasted 2 years. In 2020 the HDV infection was identified. A recent ALT determination showed its level in the normal range (35 U/l).
Patient 6
The last HDV-positive patient was a man born in 1969. The patient had multiple viral infections, such as human immunodeficiency virus (HIV), hepatitis C virus (HCV) and HBV (anti-HBc total). HIV infection was diagnosed in January 2012 at stage A1 and the patient is still taking antiretroviral drugs. The drugs prescribed were emtricitabine/tenofovir disoproxil and the complex antiviral drug cobicistat in combination with elvitegravir, emtricitabine and tenofovir. Due to HCV co-infection, PEG-IFN therapy was also introduced. Prior to hepatitis D diagnosis, the fibrosis stage was graded F4, meaning cirrhosis, which was confirmed by the biopsy. Hepatitis D was diagnosed in April 2020 as a result of expanded diagnostics due to deteriorating health. He had a FibroScan in 2022, in which the liver tissue was graded F4 (cirrhosis). A recent ALT determination indicated a slight elevation in its level – ALT 47 U/l. Other comorbidities were not noted. A risk factor for possible HDV infection was the patient’s intravenous drug use in the 1980s.
Discussion
The study demonstrates that the prevalence of the HDV virus in the Silesian region of Poland is 3.4%, which is less than the global average prevalence of around 5% [5]. In Polish research including a high-risk group of patients the prevalence was 16%, while in studies from northern and northeastern Poland it was, respectively, 4.8% and 3.9% [6, 13, 14]. There is also one Polish-based study which established the prevalence of the HDV virus at a lower level of 2.2% [7]. A detailed characterization of available data in the Polish population is presented in Table 2 and Figure 1.
Table 2
Scientific publications on the prevalence of hepatitis delta virus (HDV) in Poland
Fig. 1
Published research on the prevalence of hepatitis delta virus (HDV) in Poland. Percentage and number of HDV individuals with anti-HDV antibodies in the group of HBsAg positive subjects. *HDV-RNA-positive by RT-PCR
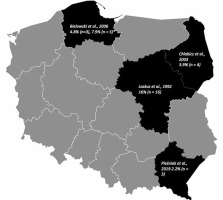
The results of this study match the general data which describe the frequency of HDV infection in surrounding countries (Austria, Belgium, Bulgaria, Czech Republic, Croatia, France, and Switzerland) as uncommon [5]. Due to the scarcity of publications about HDV in Poland, it is not possible to estimate precisely the virus seroprevalence in the national population and to compare it with our study results. However, available results do not differ from this study. Current literature suggests that patients with a history of intravenous drug abuse, originating from an HDV endemic country or laboratory findings of ALT > 40 U/l or HBV DNA < 2000 U/ml should be tested for HDV presence [15].
The diagnosis of hepatitis D is based on the assessment of HBsAg presence. Moreover, the evaluation of IgM and IgG antibodies (anti-HDV screening test), the genetic material of the virus (HDV RNA), and the viral antigen (HDV-Ag) are also important in the diagnostic pathway [16]. Unfortunately, HDV RNA testing is exceptionally uncommon in Polish laboratories, and as a result, a patient without prior contact with the virus could be underdiagnosed.
Our findings fit with the theory that HDV is the most progressive form of chronic viral hepatitis and very often progresses towards cirrhosis and liver decompensation. Two out of six infected patients required a liver transplant, and four of them presented high stages of fibrosis.
Therapy of chronic hepatitis D is a major challenge. Since the introduction of interferon α-2a treatment did not meet expectations, other drugs had to be found. Currently, there are several novel, specific anti-HDV drugs that inhibit specific stages of the HDV life cycle [17]. Bulevirtide is an entry inhibitor that blocks the binding of HBV and HDV to the NTCP (sodium taurocholate cotransporting polypeptide) protein on the surface of hepatocytes. The administration of this medicine is subcutaneous. Studies show that combining bulevirtide with PEG-IFN produces better outcomes in patients with hepatitis D. Another antiviral agent used in management of chronic HDV infection is the farnesyltransferase inhibitor lonafarnib, which is administered orally. This drug prevents the production of new HDV particles. In order to increase the positive effect of lonafarnib it has been combined with the protease inhibitor ritonavir as well as with PEG-IFN-lambda 18]. The last specific anti-HDV medications that still need further research are the nucleic acid polymers (NAPs) that inhibit HBsAg secretion [19].
The generalizability of the results is limited by the small group involved in the study and the lack of HDV RNA testing conducted due to the retrospective character of the study. Lack of RNA testing could exclude recognition of those who could have active HDV infection without prior exposure. Moreover, due to the lack of data on the exact moment of infection, we cannot determine the span of the HBV/HDV disease.
Further studies should include a larger group of patients, cover a larger area of Poland and include RNA testing. Those actions are compulsory to establish the average prevalence in the whole country. Based on the information presented, it is important to conduct large-scale clinical trials among HDV-infected patients, primarily because of the increased risk of decompensation and cirrhosis among HDV-positive patients, but also to increase knowledge of HDV seroprevalence in Poland and to introduce anti-HDV testing on a larger scale.
Conclusions
Considering that HDV infection is the most aggressive form of viral hepatitis, with the potential to lead to complete failure of this organ, the need for transplantation or even death, greater attention to this virus is required. Due to the limitations of our study, larger-scale studies addressing the question of the prevalence of HDV in Poland are needed. In addition, it is important to perform testing for HDV in patients with HBV infection. The introduction of new drugs with high efficacy against HDV infection is a milestone in improving the quality of life and health of hepatitis D patients.






