Introduction
Platinum-based compounds, such as cisplatin, carboplatin, and oxaliplatin, are extensively utilized in the treatment regimens for various malignancies, including head and neck cancers, germ cell tumors, hepatoblastomas, retinoblastomas, osteosarcomas, medulloblastomas, and hematological malignancies across both pediatric and adult populations [1]. While these compounds have significantly improved long-term survivability since their invention, they are also associated with a spectrum of adverse effects, encompassing ototoxicity, neurotoxicity, nephrotoxicity, and myelotoxicity [2].
Ototoxicity poses a significant concern, potentially resulting in permanent sensorineural bilateral hearing loss or tinnitus. Research suggests that as many as 80% of patients undergoing platinum-based chemotherapy may experience ototoxicity [3]. Several risk factors contribute to its development, including high cumulative doses of platinum-based chemotherapy, young or old age, the presence of central nervous system tumors and prior central nervous system irradiation [3].
Regrettably, there is a lack of research specifically addressing platinum-induced ototoxicity among patients with hematological malignancies. Both leukemias and lymphomas, including Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL), are diseases that can affect the pediatric and adult populations at any point of life. Given the potential ramifications of delayed detection of hearing impairment and its impact on patient quality of life, it is imperative to ascertain the incidence of ototoxicity in hematological diseases and devise guidelines for systematic audiometric monitoring in these patient cohorts.
Materials and methods
Search strategy
In order to ascertain the prevalence of ototoxicity induced by platinum-based compounds among patients with hematological malignancies, a systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [4]. Four distinct databases – PubMed (Medline), Scopus (Elsevier), Embase (Elsevier), and Web of Science – were systematically searched to identify pertinent articles for inclusion in the review. The search encompassed articles published between 2000 and 2023.
Searching and data screening
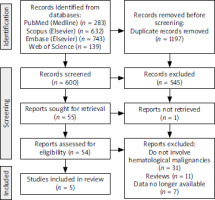
On March 13, 2023, the aforementioned databases were manually searched by three independent authors. The search utilized specific terms, including “cisplatin” OR “cisplatin-induced” OR “platinum-based chemotherapy” AND “ototoxicity” OR “ototoxic side effect” OR “ototoxic adverse effect” OR “tinnitus” AND “leukemia” OR “lymphoma” OR “hematological malignancy” OR “hematology patients”. The initial search identified a total of 1797 articles, of which 1197 duplicates were removed. Subsequently, 600 articles underwent screening, with 545 being excluded based on various criteria, such as lack of hematological patients included or focus on neurological or psychological adverse effects. Fifty-five articles underwent eligibility assessment, yet only five met the criteria for inclusion in the review. The entire screening process is delineated comprehensively in the PRISMA flowchart, presented in Figure 1. Any disparities among the three independent researchers were resolved through discussion, with inclusion of articles being contingent upon consensus.
Eligibility criteria
The systematic review adhered to a structured PICOTS (population, intervention, comparison, outcome, time, and setting) framework [5]. The population of interest comprised patients diagnosed with hematological malignancies. The intervention/exposure under scrutiny was the administration of platinum-based chemotherapy, with comparisons drawn between various platinum-compound agents such as cisplatin and carboplatin. The primary outcome of interest was the incidence of ototoxicity. Considerations were made for the temporal dimension, encompassing both the treatment phase and subsequent follow-up periods. The setting for assessment involved specialists in otolaryngology and hematologists involved in follow-up of patients with hematological malignancies. Detailed inclusion and exclusion criteria are outlined in Table 1.
Data extraction and processing
For each included article, comprehensive manual extraction of data was conducted, encompassing several key parameters: (a) study particulars, including author names and publication year; (b) total patient cohort size; (c) number of hematological patients subjected to platinum-based chemotherapy; (d) incidence of ototoxicity within the cohort; (e) treatment specifics, if provided, including the type of platinum compound administered and cumulative chemotherapy dosage; (f) available outcome measures, such as degree of hearing loss or pertinent audiometric findings. In instances where requisite information was not readily available within the main body of the research article, efforts were made to contact corresponding authors for data retrieval.
The quality assessment of included studies was executed utilizing the Newcastle-Ottawa scale [6], tailored for the evaluation of non-randomized observational studies (including cohort and case-control designs). This scale allots a maximum of nine stars, distributed across criteria pertaining to selection (four stars), comparability (two stars), and outcomes (three stars). Studies achieving a score of at least seven stars are deemed to exhibit a low risk of bias, indicative of high methodological quality.
Data availability statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at https://www.mdpi.com/ethics.
Results
Databases search results
A manual search of the mentioned databases yielded 600 results. Following the title and abstract screening, 545 articles were excluded as they did not meet the PICOTS criteria. Subsequently, 55 articles underwent full-text assessment, but 1 article could not be retrieved. Of the remaining 54 articles, 49 were excluded for various reasons: 31 did not cover auditory complications of platinum-based chemotherapy for hematological cancers, and 11 were systematic reviews. Seven research studies lacked necessary patient data and were thus ineligible for inclusion. Ultimately, 5 articles met the criteria for this systematic review.
Included studies’ characteristics
Clemens et al. conducted a comprehensive analysis involving 654 patients afflicted with various malignancies and subjected to treatment with platinum-based compounds. Among this cohort, six patients were diagnosed with lymphomas, of whom four experienced subsequent hearing impairment [7].
In a prospective follow-up study by Al-Khatib et al., 49 patients receiving platinum-based therapy were meticulously monitored, with two individuals diagnosed with lymphomas (HL and NHL). Notably, the first patient, afflicted with HL and treated with cisplatin, exhibited second-degree hearing loss, while the second patient, diagnosed with NHL and administered carboplatin, remained unaffected by hearing impairment over the observation period [8].
Moke et al. reported findings from a multi-institutional cohort study involving 1,481 patients undergoing platinum-based chemotherapy, among whom 11 were diagnosed with lymphomas (comprising both NHL and HL). Three of these lymphoma patients developed hearing impairment [9].
In a questionnaire-based investigation by Weiss et al., focusing on long-term auditory complications in children treated with platinum-based compounds, two lymphoma patients did not develop hearing loss, whereas one leukemia patient exhibited auditory deterioration [10].
Kersten et al. detailed their observations from a study involving 55 patients with relapsed and refractory classical Hodgkin’s lymphoma. Seven of these patients experienced ototoxicity, prompting a transition from cisplatin to carboplatin therapy, which notably led to hearing improvement in three cases [11].
Risk of bias assessment
Each article underwent individual scrutiny utilizing the Newcastle-Ottawa scale [6] to assess methodological quality and potential biases. Notably, all included articles demonstrated a low risk of bias. However, a significant source of bias arose due to the lack of baseline audiological assessments conducted on patients before the treatment. Therefore, it was not possible to determine whether ototoxicity occurred only because of the use of platinum-based drugs. Furthermore, several articles exhibited deficiencies in providing essential information required for this systematic review. Corresponding authors were contacted to procure the necessary data. Regrettably, in seven cases, the data were unattainable, leading to the exclusion of potentially high-quality articles from this systematic review.
Results of data synthesis
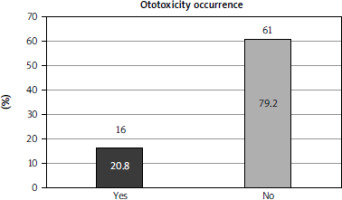
A total of 77 patients with hematological malignancies treated with platinum-based compounds were included in this systematic review. The majority of patients in the study were diagnosed with HL (65 patients, 84.4%). Nine patients were diagnosed with non-Hodgkin’s lymphoma, and only one patient had leukemia (type unspecified). The remaining two patients had lymphoma; however, the specific subtype was not specified. Ototoxicity developed in 16 patients (20.8% of the total cohort) (Fig. 2). Comprehensive data regarding the degree of hearing loss, along with all pertinent information extracted from the included articles, are presented in Table 2.
Table 2
Data extracted from the articles
| Article, year of publication | Total number of patients | Total number of patients with hematological malignancy | Total number of patients who developed ototoxicity | Hearing loss grade, name of the scale | Newcastle-Ottawa score (n) |
|---|---|---|---|---|---|
| Moke et al. [9] | 1481 | 11 | 3 | n = 1 grade 1 n = 2 grade 3 SIOP scale | 7 |
| Kersten et al. [11] | 55 | 55 | 7 | n = 3 grade 1 n = 4 grade 2 Grading scale unknown | 7 |
| Clemens et al. [7] | 654 | 6 | 4 | n = 1 grade 1 n = 1 grade 2a n = 1 grade 3a n = 1 grade 3b Muenster scale | 7 |
| Weiss et al. [10] | 2061 | 3 | 1 | Not reported | 8 |
| Al-Khatib e t al. [8] | 31 | 2 | 1 | Moderately severe hearing loss ASHA scale | 7 |
Discussion
Platinum-based agents, including cisplatin, carboplatin, and oxaliplatin, are extensively utilized in the treatment of various malignancies. Despite their efficacy, these compounds are associated with a range of adverse effects, notably including ototoxicity, neurotoxicity, nephrotoxicity, and myelotoxicity [12].
Among these agents, cisplatin is recognized as the most ototoxic. Carboplatin exhibits relatively low ototoxicity, particularly in nonmyeloablative doses. However, its administration in myeloablative doses, following cisplatin therapy, or in conjunction with osmotic agents that facilitate blood-brain barrier permeability (such as mannitol), can induce significant hearing impairment. In contrast, oxaliplatin is considered the least ototoxic agent.
A study by Hellberg et al. conducted on guinea pigs revealed a statistically significant difference in cochlear cell uptake between platinum-based compounds. Oxaliplatin demonstrated lower uptake within the inner ear compared to cisplatin, evidenced by a concentration of approximately 4 micromoles within the endolymph 30 minutes after administration, compared to cisplatin’s 14 micromoles. Consequently, oxaliplatin presents reduced ototoxic potential [13]. However, it is important to note that oxaliplatin may still induce other side effects, such as neurotoxicity and peripheral neuropathy [2].
Ototoxicity presents as a notable complication characterized by hearing loss and/or tinnitus. Initially, it affects high-frequency hearing, originating at the base of the cochlea. However, with continued exposure to platinum agents, damage to the hair cells progresses toward the apex, leading to impairment in low frequencies as well. Additionally, platinum compounds induce damage to the neurons of the spiral ganglion and stria vascularis in the cochlear duct of the inner ear [10]. Histopathological analysis of temporal bone specimens from cisplatin-treated patients confirms a gradient in outer hair cell loss, with the lowest numbers observed at the cochlear base, increasing progressively towards the midturn and apical regions [14].
Hearing impairment in the high-frequency range reduces the ability to understand voice in the presence of background noise [15] and causes difficulty in distinguishing high-frequency consonants (e.g., s, t, z, f, h, k, p) [16]. However, the pure-tone average (PTA) at medium frequencies (in the range 500–4,000 Hz) is particularly crucial for speech comprehension and daily functioning. Notably, low-frequency hearing loss is more discernible to patients compared to high-frequency loss. Among children, hearing impairment can impede speech development, contribute to educational disparities, and result in social isolation. In adults, it diminishes quality of life and independence, and may precipitate mental health issues, potentially leading to depression [17].
Furthermore, patients experiencing tinnitus endure sensations of ringing, buzzing, or hissing, which are not perceived by others. Tinnitus can significantly diminish quality of life, disrupt sleep patterns, induce anxiety, and ultimately lead to depression. However, objective assessment of tinnitus remains challenging due to the absence of validated instruments [18], and it continues to be an underrecognized long-term consequence of cancer treatment [19]. Various subjective scales, such as the visual analog scale, Tinnitus Handicap Inventory, Tinnitus and Hearing Survey, Tinnitus Functional Index, and Skarzynski Tinnitus Scale, are used to assess tinnitus severity and its impact on daily life [20]. Completion of these scales may pose difficulties for individuals with lower levels of education or children, necessitating physician assistance to elucidate questionnaire items. Nonetheless, a study by Einarsson et al. [21] revealed that up to 60% of patients included in their cohort experienced tinnitus following cancer treatment, underscoring the critical importance of monitoring patients for this distressing symptom.
Several studies have sought to elucidate the risk factors associated with ototoxicity in patients undergoing platinum-based chemotherapy. These factors encompass various demographics and treatment-related variables, including age (young age, typically less than 5 years old, or elderly), male sex, cumulative dosage exceeding 400 mg/m2, exposure to environmental noise, concurrent administration of other ototoxic medications, nutritional deficiencies, presence of anemia, and genetic predispositions [3]. In a multicenter cohort study conducted by Moke et al., additional risk factors were explored. The results showed that by increasing the drug delivery to the cochlea, a high daily dose and a high cycle dose may increase the risk of hearing loss. Notably, platinum accumulation within the cochlea is exacerbated by successive dosing regimens. Furthermore, exposure to vincristine was associated with a more than twofold increased risk of hearing impairment at the conclusion of therapy and during the latest follow-up assessments [9].
The proactive identification of patients at increased risk prior to initiating platinum-based chemotherapy holds significant potential for mitigating the incidence of ototoxicity. Evaluation of the aforementioned risk factors in each patient is essential, and consideration should be given to administering carboplatin instead of cisplatin in individuals with multiple risk factors to mitigate this risk.
Furthermore, patients presenting with multiple risk factors for ototoxicity, particularly those who will receive high-dose platinum-based chemotherapy, should undergo audiometric testing prior to treatment initiation. Patients with pre-existing hearing damage could be identified and receive a less ototoxic agent to prevent worsening of the condition. Conducting baseline hearing assessments before the beginning of the treatment facilitates early detection of ototoxicity, as subsequent test results can be periodically compared with baseline measurements. Consequently, prompt adjustments to chemotherapy regimens can be made upon the emergence of signs of hearing loss. Such interventions, including transitioning to less ototoxic agents, hold promise for averting further deterioration in hearing function and potentially facilitating full recovery.
Platinum-based chemotherapy typically does not serve as a first-line treatment for patients with lymphoma. Regrettably, even up to 50% of individuals with diffuse large B-cell lymphoma, the predominant subtype of NHL, become refractory or relapse after achieving a complete response to rituximab-based chemotherapy in combination with cyclophosphamide, doxorubicin, vincristine and prednisone [22]. In cases of disease progression during or after initial treatment, alternative chemotherapy regimens utilizing either cisplatin or carboplatin, often in conjunction with autologous stem-cell transplantation, are used. However, such approaches are accompanied by significant treatment-related toxicity [23]. In a phase II trial conducted by HOVON/LLPC Transplant BRaVE, which included 55 patients with relapsed or refractory Hodgkin’s lymphoma, seven patients experienced ototoxicity. Notably, three patients achieved full recovery following a transition from cisplatin to carboplatin therapy. Unfortunately, hearing impairment persisted in the remaining three patients, with the hearing status of one patient remaining unknown 6 months after autologous peripheral blood stem-cell transplantation [11].
The importance of audiometric testing extends beyond the treatment phase and should encompass long-term follow-up assessments, as underscored by Al-Khatib et al. in their prospective cohort study. This study monitored patients with various malignancies undergoing platinum- based chemotherapy, including two patients with lymphomas. Utilizing the American Speech-Language and Hearing Association scale, one patient with Hodgkin’s lymphoma, treated with cisplatin, developed moderately severe hearing loss, while the second patient, receiving less ototoxic carboplatin, did not experience hearing impairment. Both patients underwent long-term follow-up evaluations, revealing progression of hearing loss in the former and maintenance of hearing function in the latter over time [8]. Of all patients who underwent audiometric testing after the follow-up period, more than 30% showed high-frequency hearing deterioration after a follow-up period ranging 1.5–6.6 years after the diagnosis [8]. However, establishing a standardized endpoint for audiometric follow-up after chemotherapy remains elusive. Bertolini et al. reported cases of hearing deterioration occurring up to 136 months after treatment cessation. Thus, prolonged monitoring, potentially exceeding ten years following platinum-based chemotherapy, may be warranted [24].
According to a consensus report by the International Society of Pediatric Oncology [25], children receiving ototoxic treatments require meticulous monitoring of middle and inner ear function, along with tinnitus assessments, at each follow-up visit. Otoscopic examination, tympanometry and audiometry are recommended. In addition, it was agreed that it would be best to conduct follow-up visits before each cycle of chemotherapy; however, pre- and post-treatment testing represents the minimum standard.
Moreover, guidelines for monitoring ototoxicity in childhood, adolescent, and young adult (CAYA) patients recommend post-treatment surveillance for at least five years, commencing immediately after treatment cessation. It is proposed that audiometric testing should be performed annually for children under 6, every 2 years for children aged 6–12, and every 5 years for those over 12 [26]. Nonetheless, given the absence of guidelines for adult hematological patients, it is imperative to formulate comprehensive recommendations applicable across all age groups.
While pure-tone audiometry at 1000–8000 Hz frequencies is considered the gold standard for children over 6 years old, using multiple tests for hearing assessment is advisable [26]. Since, according to Knight et al., up to 25% of pediatric patients treated with cisplatin had ototoxicity that was not detected on a standard audiogram (which measures frequencies up to 8,000 Hz), high-frequency audiometry at more than 8,000 Hz is recommended whenever the equipment is available [27]. Patients under 6 or those presenting with symptoms of hearing loss or abnormal test results should be promptly referred to an audiologist. Despite these recommendations, the monitoring of CAYA patients remains insufficient, with only 43% of cancer survivors undergoing comprehensive evaluations before, during, and after chemotherapy [28].
Reducing disparities in diagnostic and treatment processes for adolescents and young adults is crucial. Despite the narrow age gap between pediatric patients and adolescents/young adults, the distinct treatment protocols and care settings they encounter may adversely affect prognosis. Moreover, the greater number of patients may pose challenges in terms of physician accessibility, potentially complicating the diagnosis of newly emerging hearing impairment. Consequently, individuals receiving ototoxic agents require ongoing otorhinolaryngologic monitoring for several years following treatment cessation to ensure early detection and intervention.
Patients undergoing hematopoietic stem cell transplantation (HSCT) for hematologic malignancies are susceptible to various side effects, including cardiac, renal, or pulmonary complications. According to Gertson et al., ototoxicity has been documented in 10.2% of patients after HSCT. Notably, a higher incidence of ototoxicity has been observed among patients previously treated with cisplatin compared to those treated with carboplatin before transplantation [29]. Furthermore, individuals who have undergone systemic treatment for hematologic disorders may be at risk for developing oral chronic graft-versus-host disease and secondary oral carcinoma (SOC). Secondary oral carcinoma most frequently manifests in the tongue and buccal mucosa. It is imperative that healthcare providers involved in the post-transplant follow-up of these patients remain vigilant for signs of hearing impairment and SOC. Any concerns should prompt immediate referral to an otorhinolaryngologist for further evaluation [30].
Moreover, the assessment of ototoxicity prevalence can be challenging due to the utilization of different grading scales. Clemens et al. conducted a study comparing four scales: Muenster, International Society of Pediatric Oncology Boston, Brock, Chang, and the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. Remarkably, variations in the prevalence of hearing loss ranging 33.2–51.4% were attributed solely to the use of different grading scales. Rapid detection of hearing impairment not only facilitates treatment modification but also allows for prompt introduction of aural rehabilitation. According to the authors, the Muenster scale is the most optimal for early recognition of clinically significant ototoxicity. On the other hand, ototoxicity typically begins in extended high frequencies, a feature uniquely reported in the Chang scale (up to 12,000 Hz) [7].
Conversely, some patients, even those with multiple risk factors, may not develop ototoxicity following platinum-based chemotherapy. It is essential to recognize the role of genetic predispositions in the occurrence of this adverse effect. In a recent study, Brock et al. identified 42 different variations in two genes, thiopurine S-methyltransferase (TPMT) and catechol-O-methyltransferase (COMT), strongly associated with cisplatin-induced ototoxicity in children. When combined, an individual is very likely to develop hearing loss [31]. Screening patients with multiple ototoxicity risk factors for these genetic variations could potentially enhance treatment safety. Furthermore, numerous genetic pathways responsible for the metabolism, transport, and detoxification of platinum-based agents suggest that ototoxicity may result from variations in other genes as well. As whole-genome sequencing becomes more affordable and accessible, screening all patients with ototoxicity for additional predisposing genetic variants should be considered [31].
Until such time, the extensive use of otoprotective agents remains crucial in the management of each patient. N-acetyl-cysteine and sodium thiosulfate (STS) have demonstrated efficacy in preventing hearing loss and are deemed safe for human use. Regrettably, as of September 2022, the US Food and Drug Administration (FDA) has only approved STS for the prevention of cisplatin-induced ototoxicity in pediatric patients with localized, non- metastatic solid tumors. To date, no pharmacological agents have received FDA approval for preventing ototoxicity in adults or hematological patients in general. Additionally, the transtympanic administration of corticosteroids during chemotherapy has shown promising outcomes and needs to be further investigated [32]. It is essential to initiate additional clinical trials to evaluate the efficacy of alternative potential otoprotective agents and establish optimal administration protocols [33, 34].
Regrettably, the incidence of ototoxicity among leukemia patients remains unknown due to insufficient data availability. A prospective study including patients with diverse hematopoietic malignancies undergoing platinum agent therapy is essential. Prior to chemotherapy initiation, all enrolled patients should undergo pure tone audiometry (PTA) assessment. Moreover, risk factors for ototoxicity must be evaluated in each participant. Individuals with pre-existing hearing impairments or deemed at high risk for ototoxicity should be excluded from the study. Similarly, patients with prior exposure to other ototoxic agents, such as aminoglycosides or high-dose loop diuretics, should be excluded to avoid potential bias. Subsequent PTA evaluations should be conducted after each chemotherapy cycle and annually for a minimum of five years after treatment completion. In cases of ototoxicity emergence during treatment, immediate transition from cisplatin to carboplatin is recommended. Furthermore, all enrolled patients should receive otoprotective agents proven safe in human trials. Additionally, given the significant role of genetic predispositions, genetic variant testing for TPMT and COMT genes is advisable if feasible. This comprehensive study would help to formulate optimal treatment and follow-up strategies for patients with hematological malignancies, aiming to minimize the hearing loss risk and increase overall quality of life.
Conclusions
Nowadays, the long-term survivability predictions are better than in the past due to the development of various treatment methods. However, it is extremely important to be aware of the impact of chemotherapy side effects on the patient’s quality of life.
Efforts to mitigate the incidence of ototoxicity are imperative. Given the significant contribution of genetic predispositions to ototoxicity, using otoprotective agents in the treatment of every patient is warranted. Additionally, conducting pre-treatment audiometric evaluations would be highly beneficial. When feasible, considering carboplatin instead of cisplatin for patients with multiple risk factors or pre-existing hearing impairment could help reduce the risk. Continuous monitoring of all patients receiving platinum-based compounds during and after chemotherapy, coupled with regular audiometric screening utilizing a standardized scale covering both lower and higher frequencies (> 8,000 Hz), is essential.
Measures should be taken to reduce the incidence of ototoxicity. Given the significant contribution of genetic predispositions to ototoxicity, using otoprotective agents in the treatment of every patient is warranted. Moreover, audiometric testing prior to treatment would be highly beneficial. When feasible, considering carboplatin instead of cisplatin for patients with multiple risk factors or pre-existing hearing impairment could help reduce the risk. All patients treated with platinum-based compounds need continuous monitoring during and after chemotherapy and should undergo regular audiometric screening using a standardized scale covering both lower and higher frequencies (> 8,000 Hz).
Engaging primary care physicians in post-treatment monitoring would be immensely advantageous. As frontline healthcare providers, they have frequent patient interactions and are well positioned to detect early signs of hearing impairment. Hence, raising awareness about the potential adverse effects of platinum-based chemotherapy among primary care physicians is crucial. Further research is warranted across all hematology patient cohorts to minimize treatment-related adverse effects that significantly impact quality of life.