Introduction
As an organ with immune tolerance, the liver provides an environment where tumor cells are more likely to evade immune surveillance and subsequent attack [1]. Cancer immunotherapies have been developed based on the discovery of cancer immune checkpoints that can reactivate immunocytes and contribute to antitumor immune responses [2, 3]. Due to the memory of the adaptive immune system, immunotherapy can induce a persistent response, which translates into long-term survival in a subset of patients. However, as a standard therapy for advanced hepatocellular carcinoma (HCC) [4], only a subset of patients exhibits durable responses to immune checkpoint inhibitors (ICIs) alone. Acquired resistance limits the efficacy of immunotherapy [5].
The programmed death-1/programmed cell death 1 ligand 1 (PD-1/PD-L1) axis is one of the most promising signaling pathways in HCC. Blocking this pathway can trigger enduring antitumor responses and lead to long-term remissions in cancer patients. However, only 10-30% of HCC patients respond to monotherapy with PD-1/PD-L1 inhibitors [6]. Combining ICIs with systemic and/or local therapies has been suggested as a favorable approach for managing acquired resistance [5]. The combination of ICIs and targeted drugs or interventional treatment synergistically reshapes the tumor immune microenvironment and destroys the tumor vasculature, resulting in longer progression-free survival and overall survival [7-10]. However, the characteristics of the therapeutic progression to combination treatment in patients with HCC are still unknown. Therefore, we performed a retrospective cohort study to comprehensively describe the progression patterns in patients with advanced HCC treated with a combination of local therapy, targeted drugs, and PD-1/PD-L1 inhibitors.
Material and methods
Study design and participants
Between October 2019 and April 2022, patients with advanced HCC who received local therapy combined with targeted drugs and PD-1/PD-L1 inhibitors were enrolled. The final follow-up was conducted in November 2023. HCC was diagnosed based on the radiological or histological criteria proposed by the American Association for the Study of Liver Diseases guidelines [11]. The inclusion criteria were as follows: (1) Barcelona Clinic Liver Cancer (BCLC) stage C; (2) aged 18-80 years; (3) local therapy, targeted drugs, and PD-1/PD-L1 inhibitors administered during HCC treatment; (4) no other malignancies; (5) local therapy including transarterial chemoembolization (TACE), hepatic arterial infusion chemotherapy (HAIC). The exclusion criteria were as follows: (1) fewer than two cycles of treatment with PD-1/PD-L1 inhibitors, (2) immune cell therapy. Based on disease progression, patients were divided into two subgroups. Patients in group A did not receive any treatment when diagnosed with BCLC C. Patients in group B have progressed from BCLC B to BCLC C. The Ethics Committee of the Henan Cancer Hospital approved this study (approval number: 2017003). Because of the retrospective design of study, the requirement for informed consent was waived by the Ethics Committee. All extracted data were analyzed anonymously.
Data collection and treatment regimens
Baseline demographics and clinical characteristics of the patients, including sex, age, etiology, liver function, extrahepatic lesion, alpha-fetoprotein (AFP), largest tumor size, tumor number and combination strategies, were collected from medical records. PD-1/PD-L1 inhibitors and targeted drugs were administered at standard doses, according to the manufacturer’s instructions. Dose reduction was performed for patients who experienced uncontrollable adverse reactions, and the drugs were discontinued when an unacceptable or serious adverse reaction or tumor progression occurred. Local therapy was administered to patients with local progression and/or recurrence, and targeted drugs or PD-1/PD-L1 inhibitors were adjusted for patients who had continued progression after local therapy. PD-1/PD-L1 inhibitors were discontinued when a complete response was achieved after 2 years of follow-up. The procedures for TACE and HAIC have been described in detail previously [12, 13]. All patients signed consent forms before local therapy, targeted drugs, and PD-1/PD-L1 inhibitors.
Assessment of treatment response
Contrast-enhanced dynamic computed tomography (CT) or magnetic resonance imaging (MRI) examinations were performed to evaluate the treatment response after the combination therapy every 6-8 weeks, and non-enhanced CT was performed every 3-4 weeks. For patients with a complete response or stable disease, CT or MRI was performed every 2-3 months. The treatment response was assessed according to the modified Response Evaluation Criteria for Solid Tumors (mRECIST) [14]. Intrahepatic progression was defined as an increase in the size of the primary tumor and/or the appearance of a new intrahepatic lesion after combination therapy; extrahepatic progression was defined as an increase in the size of the extrahepatic metastatic tumor and/or the appearance of a new extrahepatic lesion after combination therapy.
Disease progression was divided into the first and second stages in this study. First progression was defined as disease progression evaluated using CT or MRI after combination therapy. Second progression was defined as disease progression at subsequent follow-up after the first progression. A durable response was considered to be achieved when a complete response, partial response, or stable disease was observed after combination therapy, and disease progression was not detected during follow-up.
Peripheral blood lymphocyte analysis
Peripheral blood immune cell subpopulation analysis was performed using FACS Aria II flow cytometry and the corresponding lymphocyte subpopulation detection kit (Smultest IMK-Lymphocyte) from Becton Dickinson, USA. Specific anti-PD-1-FITC (Biogene) was used for PD-1 detection. The data analysis was completed by FlowJo software (TreeStar Inc).
Statistical analysis
Continuous variables were compared using the Mann- Whitney U test, and categorical variables were compared using the chi-square test. Statistical analyses were performed using the R software (version 3.6.1; Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism software (version 7.0; GraphPad Software, San Diego, CA, USA). Statistical significance was set at p < 0.05.
Results
Patient baseline characteristics
In total, 86 patients with BCLC stage C HCC (55 and 31 patients in groups A and B, respectively) were enrolled. Among them, 77 (89.5%) were male and 9 (10.5%) were female, with a mean age of 53.50 ±9.84 years. The main cause of HCC was viral infection, including hepatitis B virus (HBV, 93.0%) and hepatitis C virus (HCV, 3.5%). In total, 74 patients (86.0%) had liver cirrhosis, and 62 patients (72.1%) had extrahepatic lesions. Detailed information on AFP, largest tumor size, tumor number and combination strategies of target drugs and PD-1/PD-L1 inhibitors is presented in Table 1.
Table 1
Baseline characteristics of the 86 patients
Characteristics of intrahepatic progression
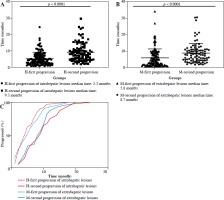
To investigate the impact of the combination therapy on disease progression, the median time to disease progression in intrahepatic and extrahepatic lesions was compared. During the analysis of intrahepatic progression, 26 of 86 patients (30.2%) did not show intrahepatic progression after combination therapy, whereas 60 of 86 patients (69.8%) showed first progression. Secondary progression was observed in 40 of 60 patients (66.7%). The median time to first progression (H-first progression) was 5.3 months for all 60 patients (95% CI: 2.3-7.1 months, Fig. 1A). The median time to second progression (H-second progression) was 9.3 months in all 40 patients (95% CI: 4.8-11.8 months, Fig. 1A).
Characteristics of extrahepatic progression
In total, 61 of 86 patients (70.9%) experienced their first progression in the extrahepatic progression cohort. Among them, 39 of 61 patients (63.9%) had a second progression. The median time to first progression (M-first progression) was 5.8 months for all 61 patients (95% CI: 1.6-8.4 months, Fig. 1B). The median time to second progression (M-second progression) was 8.7 months in all 39 patients (95% CI: 4.5-10.9 months, Fig. 1B). Based on the analysis of the time point curve of disease progression, it was observed that following combined treatment, the majority of patients initially experienced intrahepatic progression, but as treatment progressed, extrahepatic progression became the predominant trend (Fig. 1C). Of the 86 patients, only 11 (11/86, 12.8%) patients who had more than 5 months of follow-up time showed a durable response.
Characteristics of extrahepatic metastasis in the therapeutic progression
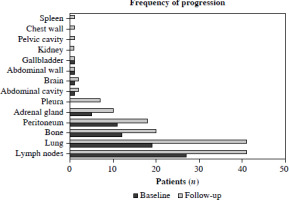
The most common sites of extrahepatic metastasis were the lymph nodes (27/62, 27.6%), lungs (19/62, 14.5%), bone (12/62, 19.4%), peritoneum (11/62, 17.7%), adrenal glands (5/62, 8.1%), abdominal cavity (1/62, 1.6%), brain (1/62, 1.6%), abdominal wall (1/62, 1.6%), and gallbladder (1/62, 1.6%). Previous research indicated that metastasis was correlated with the prognosis of HCC patients receiving combination treatment [15]; therefore, the common sites of extrahepatic metastasis were further compared during the follow-up. At the end of follow-up, the most common sites of extrahepatic metastasis were the lymph nodes (41/62, 66.1%), lungs (41/62, 66.1%), bone (20/62, 32.3%), peritoneum (18/62, 29.0%), adrenal glands (10/62, 16.1%), pleura (7/62, 11.3%), abdominal cavity (2/62, 3.2%), brain (2/62, 3.2%), abdominal wall (1/62, 1.6%), gallbladder (1/62, 1.6%), kidney (13/91, 14.3%), pelvic cavity (13/91, 14.3%), chest wall (13/91, 14.3%), and spleen (13/91, 14.3%, Fig. 2).
Characteristics of peripheral blood immune cells after combination treatment
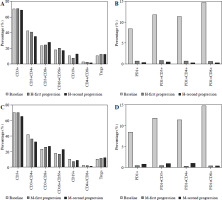
The analysis of intrahepatic and extrahepatic progression showed that most patients had disease progression within one year after combination therapy, and the lymph nodes were the most common site of metastasis, which correlated with systemic immune regulation [16]. Therefore, the changing patterns of peripheral blood immune cells were analyzed. There were three subgroups in this analysis: the time point of baseline (n = 19), the time points of first progression in intrahepatic (H-first progression, n = 29) and extrahepatic progression (M-first progression, n = 18), and the time points of second progression in intrahepatic (H-second progression, n = 17) and extrahepatic progression (M-second progression, n = 18). The results showed different trends in the changes of CD3+ T cells, CD3+CD4+ T cells, CD3+CD8+ T cells, and CD16+CD56+ cells between cases of intrahepatic and extrahepatic progression (Fig. 3A, C) and a gradual decrease in the percentages of PD1+ cells, PD1+CD3+ T cells, PD1+CD4+ T cells and PD1+CD8+ T cells following combination therapy, which gradually increased during the follow-up in cases of extrahepatic progression (Fig. 3B, D).
Fig. 3
The change of peripheral blood immune cells after combination treatment. A) The change of CD3+ T cells, CD3+CD4+ T cells, CD3+CD8+ T cells, CD16+CD56+ cells, CD19+ cells, CD4+ T cells/CD8+ T cells, Tregs in the intrahepatic progression cohort. B) The change of CD3+ T cells, CD3+CD4+ T cells, CD3+CD8+ T cells, CD16+CD56+ cells, CD19+ cells, CD4+ T cells/CD8+ T cells, Tregs in the extrahepatic progression cohort. C) The change of PD1+ cells, PD1+CD3+ T cells, PD1+CD4+ T cells, PD1+CD8+ T cells in the intrahepatic progression cohort. D) The change of PD1+ cells, PD1+CD3+ T cells, PD1+CD4+ T cells, PD1+CD8+ T cells in the extrahepatic progression cohort
Discussion
This study describes the clinical characteristics of disease progression in patients with stage C BCLC HCC after local therapy combined with targeted drugs and PD-1/PD-L1 inhibitors. For the first stage of cancer progression, the results showed that the median time of intrahepatic progression was shorter than that of extrahepatic progression. For the second stage of cancer progression, the results showed that the median time of extrahepatic progression was shorter than that of intrahepatic progression. The common sites of extrahepatic progression are the lymph nodes and lungs. Analysis of peripheral blood immune cells showed a decrease in several types of immune cells after combination treatment, followed by a gradual increase during follow-up, especially for PD1+ cells in extrahepatic progression.
Treatment with ICIs has been suggested for several types of cancer. However, most patients do not benefit from ICI treatment alone. The response rate to single-agent PD-1 inhibitors is in the range 15-32% in HCC [17-20] and 10-70% in other types of cancer [21]. Even among patients who initially respond to ICIs, disease progression can eventually occur, and only a minority of patients achieve long-term durable responses. To overcome resistance, combination strategies have been suggested to broaden the response population [5, 21]. Triple therapy, which includes TACE, multitarget drugs, and PD-1 inhibitors, has been reported to enable further improvement in patient progression-free and overall survival in advanced HCCs [10, 14, 15, 21-23]. However, the therapeutic response was still poor in the combination treatment of advanced HCC patients, and our results showed that only 12.8% of patients received a durable response during follow-up, which was lower than previously observed [10, 15]. This discrepancy may be attributed to our long-term follow-up.
Recent studies have reported that cancer patients with liver metastases demonstrate significantly worse outcomes than those without liver metastases when treated with immunotherapy. Liver metastases can induce a reduction in systemic antitumor immunity through the coordinated activation of Tregs and CD11b+ monocytes [24] and the systemic loss of CD8+ T cells [25]. Local therapy can induce local inflammation, promote antigen release and immune cell infiltration, and a hypoxic microenvironment can induce angiogenesis. Common multitarget drugs can target numerous proteins to suppress tumor angiogenesis and kill tumor cells. However, the characteristics of progression-related immune responses in patients with HCC were unclear. Our results showed a gradual decrease in the percentages of PD1+ T cells, PD1+CD3+ T cells, PD1+CD4+ T cells and PD1+CD8+ T cells following combination therapy, which gradually increased during follow-up in cases of extrahepatic progression. A previous study showed that a decrease in PD1+ T cells in the peripheral blood after peptide vaccine therapy was correlated with longer overall survival in lung cancer [26]. Also, higher expression levels of PD-1 on CD3+ T cells and on CD8+ T cells were found to negatively impact patients’ clinical response and survival [27], which was consistent with our study. However, the mechanism by which these immune cells contribute to disease progression or prolonged survival requires further study. In this study, the lymph nodes and lungs were the most common sites of disease progression. Generally, the bloodstream and direct cancer cell invasion are the primary routes for extrahepatic metastasis in patients with HCC. The frequent site of extrahepatic metastatic HCC include the lungs, bone, lymph nodes, and adrenal glands [28, 29]. The most common metastatic sites are the lymph nodes and lungs after sorafenib treatment as first-line therapy [30]. Additionally, our results showed that the most common sites of disease progression were the lymph nodes (66.1%) and lungs (66.1%) during follow-up. A recent study reported that the lymph node is not a passive post-metastasis stage but is a critical site for inducing systemic immunosuppression. It promotes metastasis by inducing tumor-specific immune tolerance, and can alter the systemic immune response by inducing tumor-specific Tregs, increasing PD-L1 expression in macrophages, and shifting dendritic cells (DCs) from migratory to resident subtypes [16]. In the present study, immune response analysis showed that several types of immune cells were altered after combination treatment; therefore, the lymph nodes as the main site of metastasis may be related to changes in the immune response induced by local therapy and PD-1/PD-L1 inhibitor treatment.
This study has several limitations. First, this was a retrospective study, and the combination strategies were not uniform among the patients. Second, the sample size of this study was limited. Third, the follow-up time was short in this study; therefore, the overall survival was not evaluated.
Conclusions
The clinical characteristics of intrahepatic and extrahepatic progression were different in patients with BCLC stage C HCC after local therapy combined with multi-target drugs and PD-1/PD-L1 inhibitors. The intrahepatic and extrahepatic progression exhibited distinct patterns in terms of the median time of disease progression and peripheral blood immune cells. Lymph nodes and lungs were the most susceptible sites for disease progression, which may also be correlated with changes in the immune response after combination treatment.