Introduction
Skin inflammatory and allergic conditions often manifest with symptoms such as erythema, oedema, and pruritus. Allergic reactions may be caused by external factors such as insect bites, contact with plants (e.g. nettle, ivy) and with marine organisms and can have negative consequences on quality of life and daytime function [1, 2]. Insect bites may cause a local reaction that is usually pruritic and consists of local erythema and oedema, a typical wheal and flare response [1, 3]. Physical contact with numerous tiny needles like hairs present on leaves and stems of stinging nettle (Urtica dioica) may result in a contact urticarial dermatitis due to chemical and mechanical irritation triggered by skin penetration of the hairs containing histamine [4, 5]. The venoms of various animals such as coelenterates, octopuses, spiders, scorpions, centipedes, and insects contain histamine. Additionally, many animal venoms contain compounds that induce release of histamine from mast cells [6]. Jellyfish tentacles in contact with human skin can produce pain, swelling and redness, due to discharge of jellyfish nematocysts and associated toxins [7].
Most insect stings cause mild local reactions for which no specific treatment is usually required. Due to the similar mechanism of induction of local reactions by nettle stingers (allergic and inflammatory reaction in response to injected foreign substances and puncture of stinging hairs), local management of mild inflammatory/allergic reactions following contact with stinging plants should be considered similar to a mild reaction to insect bites [5]. Treatment of jellyfish stings in humans traditionally include acetic acid (vinegar), sodium bicarbonate (baking soda), ammonia, papain or bromelain (meat tenderizer), ethanol and salt water. Diluted solutions of local anaesthetics, e.g. benzocaine, lidocaine, have been recommended to bring relief from jellyfish stings [7]. Antihistamines and analgesics may help to reduce the pain or itching associated with cutaneous reactions.
In order to address treatment of histamine-dependent allergic and inflammatory skin reactions, a combination of the active substances, diphenhydramine and lidocaine, was developed. Allefin gel is a topical medicinal product indicated for use in adults, adolescents and children of 2 years of age and older for the symptomatic treatment of contact allergic and inflammatory skin lesions accompanied by pruritus, responsive to treatment with antihistamines and arising as a result of external factors, such as bites and stings (e.g. insects, arachnids), contact with certain plants (e.g., nettle, ivy), burns by jellyfish [8]. The complex activity of this combination in the symptomatic treatment of local pain and pruritus is based on the different mechanisms of action of the two active substances in controlling the symptoms of contact dermatitis. Diphenhydramine, a first-generation antihistamine, possesses potent antiallergic properties by inhibiting histamine receptors (H1) and was shown to be effective in treating urticaria [9]. On the other hand, lidocaine, a local anaesthetic, exhibits analgesic effects and alleviates pruritus by blocking the subpopulation of sensory neurons [10]. The onset of action of lidocaine is less than 2 min when applied to the skin, the duration of anaesthesia is 30–45 min [10, 11]. The synergistic effects of diphenhydramine and lidocaine hold potential in providing a comprehensive treatment option for individuals with inflammatory and allergic skin conditions, who often experience the distressing sensation of pruritus alongside other symptoms. In the literature, numerous publications describe the beneficial role of these two single compounds in the treatment of reactions to insect and arachnid bites and stings, contact with specific plants like nettle and ivy, and jellyfish burns [6, 12–16].
Aim
The aim of study was to evaluate efficacy and safety of the combination topical gel containing diphenhydramine hydrochloride 20 mg/g and lidocaine hydrochloride 10 mg/g over placebo in the treatment of local skin inflammatory and allergic reactions.
Material and methods
The study was approved by the Independent Ethics Committee in Poland (no. KB/1220/19) and was registered in EudraCT (no. 2018-004502-26). All the procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2000. It was a single-centre, single-dose, randomized, double-blind, two-treatment, two-period, two-sequence (each forearm) cross-over design study with a wash-out period of minimum 46 h. Topical product administration in the study group of forty-four (44) healthy volunteers who met all of the inclusion and none of the exclusion criteria (including histamine screening skin test) was performed. The subjects were ≥ 18 and ≤ 55 years of age. All of study participants were included in the safety analysis and 39 of them were statistically evaluated for the efficacy/pharmacodynamic parameters. A design for comparing two formulations, consisting of two sequences and two periods was used (test – placebo or placebo – test). The tested product was diphenhydramine hydrochloride and lidocaine hydrochloride, 20 mg/g and 10 mg/g, gel (0.16 ml of a product – single topical application) and the comparative product was Placebo, gel (0.16 ml of a product – single topical application). Safety monitoring was done during all study periods.
The primary endpoint was the difference in area under the curve (AUC) calculated from the intensity of itch for test product (A) and placebo product (B) assessed using a visual analogue scale (VAS) at each estimation time point. The secondary endpoints included evaluation of change in diameter of the wheal and the erythema, rate of decrease in itching, peak itch intensity and recording of all adverse events (AE).
Itching assessment
Evaluation of itch was performed using the VAS scale, after histamine administration (time “0”) and at: 2, 4, 6, 8, 10, 15, 20, 30, 60 and 90 min after test product/placebo administration. The intensity of the elicited itch was assessed from the moment of application, using a VAS. The obtained results were compared between groups (test product vs placebo product). The VAS ranged from 0 = “no itch” to 10 = “worst imaginable itch” with a label at 50% of its length, labelled “strong urge to scratch”. Similar VAS modifications were used in the study by Hartmann et al. [17]. The subjects were instructed to rate the sensation of itch by using the 50% mark as a guideline.
Wheal/erythema assessment
Evaluation of the diameter of the wheal and the erythema was performed after histamine administration (time “0”) and at: 2, 4, 6, 8, 10, 15, 20, 30, 60 and 90 min after test product/placebo administration. Two diameters of the wheal and the erythema were measured (the longest diameter and the diameter perpendicular to it) with the use of a ruler. The diameters were recorded in millimetres. The obtained results were compared between groups (test product vs placebo product).
Safety
During the study all subjects were under close observation by the investigators and the nursing staff to assure maximum safety and to collect all AEs. During the wash-out period subjects could contact the principal investigator or other medical staff if they felt it was necessary.
Statistical analysis
It was planned to include 44 subjects into the study. It was assumed that drop-out would be about 10%, thus 40 subjects were to be included into analysis. Justification for this sample size was made under the assumption that analysis of the primary endpoint was done using paired t-test with a level of significance of 0.05 and power of 80%. If standard deviations of mean difference were of size of about 30% of the mean value of AUC (assumption based on results reported by Andersen et al. [18]), with the sample size of 40 the smallest possible difference to detect as statistically significant was of size of 13.6% of the mean value of AUC in the placebo group, which was enough for achieving the study objective.
Analysis of AUC was conducted using the multivariate linear mixed model with fixed effect of period, sequence and product as well as random subject effect. Comparison of AUC was done using paired t-test and separately using logarithmic transformation and non-parametric paired Wilcoxon test. The significance levels were set at 0.05. Analyses were performed using SAS software. All subjects exposed to the study products were a part of the safety analysis.
Results
Baseline characteristics of study participants
In period 1, 44 subjects (20 males and 24 females) who fulfilled the eligibility criteria (all inclusion and none of the exclusion criteria) have been assigned a randomization number, according to the order of arrival at the site. All were judged to be healthy, based on medical history, physical examination and clinical laboratory tests. The total of 44 and 43 subjects were administered the test or placebo product on day “1” of period 1 and 2, respectively. One subject did not appear at the clinical site for drug administration in period 2. Additionally, four (4) subjects did not respond with itching after histamine administration in period 2. In consequence, forty-three (43) subjects completed the whole study (periods 1 to 2) and thirty-nine (39) subjects were included into statistical analysis.
Statistical analysis of demographic data of 44 subjects (20 males and 24 females), who were randomized and dosed, is shown in Table 1.
Primary endpoint of the study: difference in itching AUC
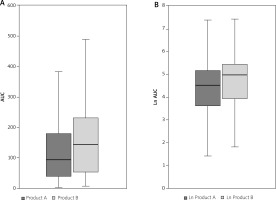
In terms of the primary endpoint itching AUC was significantly greater for product B (placebo) than for product A (test product). This was confirmed both in the univariable analysis (AUC A vs. B: 185.7 ±289.3 vs. 301.5 ±504.4, Wilcoxon signed rank test p-value for paired data: p = 0.017) as well as in logarithmically transformed itching AUC (A vs. B: 4.4 ±1.4 vs. 4.8 ±1.4, Student t-test p-value for paired data: p = 0.024). Results of primary variable analysis are shown in Tables 2 and 3 and Figure 1.
Table 2
Comparison of itching AUC between products (Wilcoxon signed rank test p-value for paired data: 0.017)
| Itching AUC | Test product (A) | Placebo (B) |
|---|---|---|
| N | 39 | 39 |
| Mean (SD) | 185.7 (289.3) | 301.5 (504.4) |
| Median [Q1–Q3] | 92.5 [37.0–177.8] | 143.0 [51.0–230.0] |
| Min.–max. | 1.0–1615.5 | 5.0–2276.0 |
Table 3
Comparison of logarithmically transformed itching AUC between products (Student t-test p-value for paired data: 0.024)
| Itching AUC | Test product (A) | Placebo (B) |
|---|---|---|
| N | 39 | 39 |
| Mean (SD) | 4.4 (1.4) | 4.8 (1.4) |
| Median [Q1–Q3] | 4.5 [3.6–5.2] | 5.0 [3.9–5.4] |
| Min.–max. | 0.0–7.4 | 1.6–7.7 |
Figure 1
A – comparison of itching AUC – box plot illustrating median AUC, boundaries of the box signify the lower and upper quartiles (Wilcoxon signed rank test p-value for paired data: 0.017). B – comparison of logarithmically transformed itching AUC – box plot box plot illustrating median ln AUC, boundaries of the box signify the lower and upper quartiles (Student t-test p-value for paired data: 0.024)
Secondary endpoint: change in diameter of the wheal and the erythema
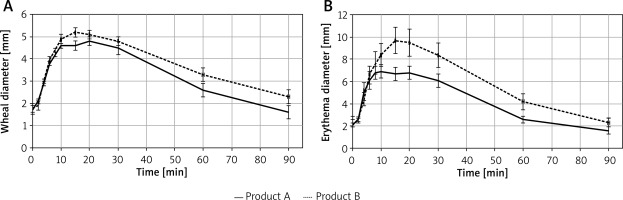
Wheal diameter and area, and erythema diameter and area were analysed descriptively and using the linear mixed model, with fixed effects for product and time and random intercept for subject. Descriptive analysis did not reveal clear differences between products, but the results of the analysis performed using linear mixed models showed that the symptoms reported for product B were more severe than for product A and the product effect was statistically significant. Wheal diameter was higher for product B (placebo), on average by 0.31 mm, and wheal area – by 1.80 mm2. Erythema diameter was higher for product B (placebo) on average by 1.2 mm, and erythema area – by 19.16 mm2. The visible effect was observed 15 min after application of the test product. The results regarding wheal and erythema diameter in consecutive time points are presented in Figure 2.
Figure 2
A – Mean (± SEM), wheal diameter value in consecutive time points. B – Mean (± SEM), erythema diameter value in consecutive time points
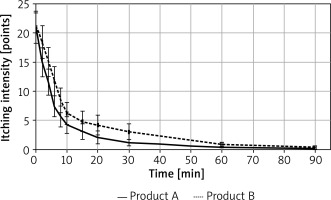
Itching intensity was higher for product B (placebo), on average by 2.05 (10-point VAS scale was used), and itching intensity decreased with time (average rate of decrease was 0.168/min, 95% CI: 0.143–0.192/min). A decrease in itching intensity was observed from the second minute after application of the test product. The results are presented in Figure 3.
Secondary endpoint: peak itch intensity
Peak intensity depending on time and the product was as follows: test product (A) vs placebo product (B): 22.8 ±16.6 vs. 24.9 ±17). However, in univariable analysis, no significant differences in peak itching intensity between products was identified (Wilcoxon signed rank test p-value for paired data: 0.311).
Discussion
Results of the presented clinical trial show evidence for efficacy and safety of lidocaine and diphenhydramine combination in treatment of histamine-related conditions (i.e. contact inflammatory and allergic skin reactions, accompanied by pruritus). Such results are in line with available literature data. Lidocaine belongs to the group of medicines called local anesthetics. Its anaesthetic mechanism of action involves blocking signals at nerve endings in the skin. Lidocaine is used to relieve pain and itching caused by e.g. insect bites or stings. The anaesthetic efficacy of topical lidocaine products used before injection, in chronic pain, skin infections (herpes viruses), sunburns, insect bites, burns from contact with plants, small wounds, scratches, during intubation, before diagnostic ophthalmic procedures, dental, laryngological and dermatological procedures has been reported in multiple studies and reviews [19–27]. In this study, a visible difference in the intensity of itching was observed already in the second minute of the examination, which is related to the effect of lidocaine and is consistent with literature data. There were no significant differences in peak itching intensity between test products and placebo (p = 0.311), but lack of statistically significant difference in peak itching intensity was expected while pick itching was generally observed shortly after the application of histamine. This also confirms that the initial condition of the subjects was comparable. The onset of action of lidocaine is less than 2 min when applied to the skin, the duration of anaesthesia is 30–45 min [10, 11]. A study published by Birsa et al. [7] has shown that lidocaine in concentrations of 1–3% reduces burning caused by contact with jellyfish within 10 to 20 min from application, and also reduces the formation of swelling and redness of the skin at the point of contact with stings. Higher concentrations of lidocaine may alleviate pain symptoms within the first minute of the topical application [28]. Itching intensity observed in this study was higher for placebo, on average by 2.05 (10-point VAS scale). Descroix et al. [29] performed double-blind, randomized, placebo-controlled, six-centre trial on 59 patients in which the application of the 1% lidocaine cream led to a mean reduction in VAS pain intensity of 29.4 ±17.0 mm, which was significantly greater than the decrease obtained with the placebo cream (p = 0.0003).
Diphenhydramine as a H1 receptor antagonist, reduces allergic symptoms related to histamine secretion. McGavack et al. [30] have shown that in 63 subjects the topical application of 2% and 5% concentrations of diphenhydramine hydrochloride (Benadryl) in ointment bases reduced or completely destroyed the response of the skin to intradermally applied histamine. Bernstein et al. [28] investigated the antipruritic activity of topically applied 5% solutions of doxepin hydrochloride and amitriptyline hydrochloride, 5% solution of diphenhydramine and found that all of them generated significantly higher mean and median histamine itch thresholds (p < 0.01 than control vehicle). The onset of antiallergic action of diphenhydramine after application to the skin occurs after a few minutes, which was observed as visible differences in the diameter and area of the wheal and erythema 15 min after the examination. Some studies also reported anaesthetic action of topical diphenhydramine hydrochloride. In a double-blind study performed by Gallo and Ellis [31] patients with allergic reactions to general local anesthetics were effectively treated with diphenhydramine as an anaesthetic alternative. The local anaesthetic efficacy of diphenhydramine to other more commonly used agents for dentistry was investigated in two clinical trials. Meyer and Jakubowski [32] performed a double-blind study of tooth extractions using either 1% diphenhydramine or 2% lidocaine with 1 : 100,000 epinephrine. Nine patients received diphenhydramine and seven received lidocaine. Although all 7 patients who received lidocaine experienced complete anaesthesia, only 4 of the 9 patients receiving diphenhydramine experienced complete anaesthesia. Welborn and Kane [33] compared 1% diphenhydramine with 1 : 100,000 epinephrine to 2% lidocaine with 1 : 100,000 epinephrine in 50 patients undergoing extraction of mandibular third molars. The onset of anaesthesia took considerably longer when diphenhydramine was used. The duration of anaesthesia with diphenhydramine was shorter than with lidocaine and a larger concentration of diphenhydramine was necessary. 48% of the patients receiving diphenhydramine and 64% of the patients receiving lidocaine experienced complete anaesthesia. The prospective study conducted by Ernst et al. [34] compared the effectiveness of 1% diphenhydramine with 1% lidocaine for local anaesthesia in repair of minor skin lacerations in adults aged 18 to 64 years with simple linear lacerations. According to patient rating, lidocaine was less painful for injection than diphenhydramine (p = 0.0017).
To the authors’ best knowledge, until now the combinations of lidocaine and diphenhydramine have been tested only in treatment of mucositis-induced discomfort in patients receiving chemotherapy. Turhal et al. [35] performed small-scale clinical trial (n = 31), which results suggest that the three-drug mouthwash (lidocaine, diphenhydramine and sodium bicarbonate in normal saline) provides effective symptomatic relief in patients with chemotherapy-induced mucositis. A phase 3 placebo-controlled clinical trial (n = 275) showed a significant reduction of mucositis pain for both diphenhydramine-lidocaine-antacid mouthwash and doxepin mouthwash in patients undergoing head and neck radiotherapy [36].
The study provided evidence for differences in efficacy between the tested product and placebo. The results revealed that itching intensity AUC was significantly greater for placebo. No significant differences between products in peak itching intensity were identified. However, symptoms reported for product B were more severe than for product A and the product effect was statistically significant. Therefore, one can conclude that product A (test product) more effectively reduced the inflammatory and allergic symptoms than product B (placebo). The clinical results demonstrated that the safety profile of test product (A) was similar to placebo.
Conclusions
The presented clinical study justifies the use of the combination of diphenhydramine hydrochloride with lidocaine hydrochloride in histamine-dependent inflammatory and allergic skin reactions accompanied by itching. It was shown that the combined medicinal product applied topically reduce itching and other signs of the histamine reaction (wheal and erythema) in relation to placebo in a model of a local histamine-induced allergic reaction in healthy volunteers.