Introduction
Just a few years ago, the hepatitis C virus (HCV) infection in hemodialyzed patients suffering from chronic kidney disease (CKD) had a very significant impact on their outcome [1]. Renal replacement therapy increased the risk of virus transmission. On the other hand, HCV untreated for many years led to the increase of proteinuria and ultimately the progression of the condition to its final stage which requires renal replacement therapy [2, 3]. Metabolic complications such as diabetes, arterial hypertension, obesity, or lipid disorders occur more often in patients infected with HCV. They are independent risk factors for the progression of the patient’s kidney disease and cardiovascular complications, the main cause of high mortality among CKD patients [4, 5]. In times of interferon (IFN) and ribavirin (RBV), the therapeutic possibilities and their effectiveness were limited [6]. The complications of an ongoing chronic HCV infection, increasing prevalence due to progressing fibrosis or cirrhosis, as well as various extra-hepatic manifestations, and also being disqualified from transplant procedures, were responsible for the shorter life expectancy of CKD patients infected with HCV. They also left the patients with a feeling of social isolation [7]. The later improvements in sanitary oversight, adherence to sterilization rules, control over blood donors, and early detection have significantly lowered HCV transmission among the population which was once considered a group of increased risk. The real breakthrough came with new treatment possibilities, which were provided by direct antiviral agents (DAA) and their subsequent generations, which have nearly 100% antiviral effectiveness and an improved safety profile [8, 9]. Not only do these drugs have an influence on the prognosis for the entire HCV-infected population, but they also contribute to the microelimination of infections and allow for the eradication of HCV on a global scale [10–12].
This observational and multicenter study aims to summarize the effectiveness and safety of genotypespecific and pangenotypic HCV treatments in patients in various stages of renal failure, hemodialyzed, and after kidney transplantation. The study takes into account the stage of liver fibrosis, genotype (GT), decompensation, comorbidities, and drugs taken by patients. The secondary aim is to optimize the selection of a therapeutic regimen depending on the availability and financial possibilities of a given center.
Material and methods
Study population
In July 2015 the Polish Association of Epidemiologists and Infectiologists initiated the EpiTer-2 project, which includes 22 hepatology centers in Poland diagnosing and treating chronic hepatitis C. Patients are treated according to the current guidelines of global hepatology associations and following the therapeutic program financed by the National Health Fund [13–15]. The data are available only to doctors participating in the project and are archived using a web-based questionnaire.
This made it possible to identify 593 patients among over 15 thousand who were entered into the database and according to the KDIGO 2022 definition fulfilled the criteria for CKD related to the kidneys being malformed or malfunctioning. Patients were divided into two groups: those treated with genotype-specific regimens (n = 428) and with pangenotypic options (n = 165), and concerning the kidney disease dependent on the stage of renal failure determined using glomerular filtration rate (GFR; Cockcroft and Gault equation): G1 and G2 – GFR ≥ 60 ml/min/1.73 m2, G3 – GFR 30-59 ml/min/1.73 m2, G4 and G5 – GFR ≤ 29 ml/min/1.73 m2. Two separate groups were created for hemodialyzed patients and patients after kidney transplantation. Apart from basic information such as age, gender, body mass index (BMI), and comorbidities, the study also included information on the extrahepatic manifestations (cryoglobulinemia, thyroid dysfunction, thrombocytopenia unrelated to liver cirrhosis) and drugs (proton-pump inhibitors, statins, anticoagulants), which due to interactions with other drugs could potentially influence the effectiveness of antiviral treatment or cause increased adverse effects (Table 1). The patients receiving the regimen containing ritonavir routinely had their calcineurin inhibitor doses modified, with control of tacrolimus through levels, before and during antiviral treatment.
Table 1
Baseline characteristics of patients
Table 2 shows the parameters for liver disease: genotype, stage of fibrosis, percentage of patients in B and C class according to Child-Pugh (CP) classification in patients with cirrhosis, as well as details of decompensation history, the occurrence of hepatocellular carcinoma (HCC), HBV (HBsAg+) and HIV coinfection, and liver transplantation.
Table 2
Characteristics of liver disease
The stage of liver fibrosis was measured with non-invasive methods: transient elastography using FibroScan (Echosens, Paris), or shear wave elastography using Aixplorer (Supersonic, Aix-en-Provence). The cut-off values for individual fibrosis levels were presented in accordance with the European Association for the Study of the Liver recommendations [14, 15].
The choice of antiviral treatment was decided upon by the physician based on the recommendations and drug reimbursements on offer in a given period from the National Health Fund.
The occurrence of a sustained virological response (SVR) was evaluated at least twelve weeks after the end of treatment (EOT) by the assessment of the ribonucleic acid (RNA) of HCV. Results were interpreted as “target not detected” (TND) meaning undetectable viral load and “target detected” (TD) indicating detectable HCV RNA. Patients who then still had a detectable viral load were categorized as virologic nonresponders.
Throughout the treatment course and a 12-week follow-up period, safety data were collected. This included information on adverse events (AEs), as well as any occurrences of deaths. Additionally, the data encompassed details about modifications or discontinuations of the therapy course when necessary.
Ethical considerations
Treatment of patients with drugs registered in Poland and under the therapeutic program did not require the approval of the Bioethics Committee. This was according to the pharmaceutical regulations at the time (Law of September 6, 2001, Pharmaceutical Law, art. 37al).
Statistical analysis
Categorical data were presented as numbers and percentages. Group comparisons were performed using Pearson’s χ2 test or Fisher’s exact test (as appropriate). Continuous data (age, BMI, and laboratory markers) were summarized by the median and interquartile range (IQR). The data did not meet the Gaussian distribution; they were checked with the Shapiro-Wilk test, and differences between the groups were assessed using the nonparametric Mann-Whitney test. In the multiple comparisons, the Bonferroni correction was applied. The SVR was evaluated for all patients who initiated the treatment as intent-to-treat (ITT) analysis and after the exclusion of those lost to follow-up as per protocol (PP) analysis. P-values of < 0.05 were considered to be statistically significant. All statistical analyses were performed using Statistica v. 13 (StatSoft, Tulsa, OK, United States) and GraphPad Prism 5.1 (GraphPad Software, Inc., La Jolla, CA).
Results
Characteristics of the study group
Five hundred and ninety-three patients with chro-nic kidney disease were identified in the EpiTer-2 database, including 134 undergoing hemodialysis and 88 after kidney transplantation. In 99 of those patients (15.7%) extrahepatic manifestations of HCV were identified, most often cryoglobulinemia (n = 65; 65.65%). Comorbidities such as arterial hypertension (67.11% of patients), cardiovascular diseases (18.21%), diabetes (27.31%), and dyslipidemia (4.21%) are typical complications or the causes of chronic kidney disease. More than 85% of patients in both groups were taking other medications: anticoagulants (20%), proton-pump inhibitors (17.6%), and statins (6.7%), which due to drug interactions could influence the antiviral response and lead to increased adverse effects. The basic characteristics of the patients are presented in Table 1.
In total, 467 patients were treatment-naïve, 75.5% and 87.3% in genotype-specific and pangenotypic groups of patients respectively, 22.2% and 28.5% had liver cirrhosis, of whom 23.2% and 36.2% were scored at baseline as B in the CP classification, with single persons in each group in class C (data are shown in Tables 2 and 3). Four hundred and twenty-eight patients received genotype-specific regimens, and 165 patients were treated with pangenotypic options (detailed information shown in Table 3).
Table 3
Treatment characteristics of infected patients
In both groups, the dominant genotype was GT1b (87.4% and 60.6% in the genotype-specific and pangenotypic regimens, respectively). Pangenotypic treatment was given to 25.5% of patients with GT3 and 9.1% with GT4.
Attention should be paid to the relatively low percentage of patients with a history of HCC (n = 24, 17% of all patients with cirrhosis). Nearly 8% of patients underwent liver transplantation in the past, which introduced prolonged treatment with a calcineurin inhibitor as an additional factor contributing to kid-ney damage.
Treatment regimens
In the population of patients treated with genotype-specific regimens, ledipasvir (LDV)/sofosbuvir (SOF) ± RBV, ombitasvir (OBV)/paritaprevir (PTV)/ritonavir (r) + dasabuvir (DSV) ± RBV and grazoprevir (GZR)/elbasvir (EBR) ± RBV treatments were given to 31.5%, 31.5% and 34.8% patients respectively. In pangenotypic regimens, glecaprevir (GLE)/pibrentasvir (PIB) was the most common option (50.3%). This regimen, along with the combination of SOF/velpatasvir (VEL) with or without RBV, represented “new” pangenotypic options. Seventeen patients in the first years of the DAA era received SOF + RBV, which was considered an “old” pangenotypic option.
In pangenotypic regimens the most often administered option was GLE/PIB (50.3%). Seventeen patients in the first years of DAA era received SOF + RBV.
In the genotype-specific treatments, the LDV/SOF ± RBV regimen was most often chosen in patients with GFR ≥ 30 ml/min, while in patients with GFR < 30 ml/min (6.7%) drugs with potentially lower nephrotoxicity were more often included.
Antiviral treatment effectiveness
Ninety-six percent of patients in the genotype-specific treatment arm and 88.5% of patients who received the pangenotypic regimens (including the “old” SOF + RBV regimen) achieved SVR12 in the ITT analysis (Fig. 1). Of the 569 patients available for evaluation, 98% (n = 556) achieved SVR12 (per protocol) – 98.9% (410/415 patients) of those treated with genotype-specific regimens and 94.8% (146/154 patients) treated with pangenotypic drugs. It needs to be stressed, however, that in the pangenotypic treatment arm, the SOF + RBV regimen was taken into account, in which 81.3% of patients achieved SVR12 (13/16 patients). If the patients treated with SOF + RBV are excluded, the “new” pangenotypic regimens allowed 96.4% of patients to achieve SVR12 (Fig. 1). Of the 415 patients treated with genotype-specific regimens (PP analysis), 5 did not respond to the antiviral treatment, of which 3 were treated in the OBV/PTV/r + DSV ± RBV regimen and 1 in the GZR/EBR ± RBV and asunaprevir (ASV) + daclatasvir (DCV) regimens.
Fig. 1
Therapy effectiveness by treatment regimen with comparison of genotype-specific vs. pangenotypic options, and between pangenotypic old (SOF + RBV) and new (GLE/PIB and SOF/VEL + RBV) regimens according to the intention-to-treat (ITT) and per protocol (PP) analysis
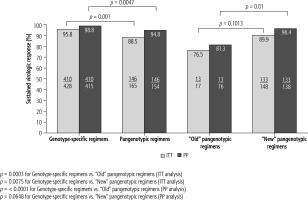
Virologic failure was noted in eight patients of the 154 treated with pangenotypic regimens, of which four were treated with SOF/VEL ± RBV, three with SOF + RBV, and one with GLE/PIB (Table 4). The addition of RBV to the antiviral regimen did not increase the percentage of virological response.
Table 4
Comparison of virological responders and nonresponders to direct-acting antiviral therapy
[i] ASV – asunaprevir, BMI – body mass index, DCV – daclatasvir, DSV – dasabuvir, EBR – elbasvir, F – fibrosis stage, GLE – glecaprevir, GT – genotype, GZR – grazoprevir, HCC – hepatocellular carcinoma, IQR – interquartile range, KTx – kidney transplantation, LDV – ledipasvir, OBV – ombitasvir, OLTx – orthotopic liver transplantation, PIB – pibrentasvir, PPI – proton-pump inhibitors, PTV/r – paritaprevir, RBV – ribavirin, SOF – sofosbuvir, VEL – velpatasvir
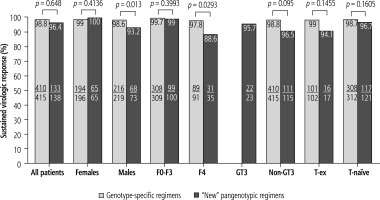
When comparing patients infected with GT3 HCV to the non-GT3 population, it can be seen that the first did not respond to antiviral treatment significantly more often than the latter (30.8% vs. 6.1%). Similarly, the treatment failed statistically significantly more frequently in patients with the most advanced fibrosis and cirrhosis (61.5% in F4 vs. 23.1% in the case of fibrosis F0-F3 and 61.5% in patients with F3-F4 vs. 23.1% in F0-F2). Among the patients with more advanced kidney failure (G4-G5), hemodialyzed, and after kidney transplant, 100% responded to the antiviral treatment (Fig. 2). A comparative analysis of effectiveness showed no difference based on the type of DAA regimen, while an additional sub-analysis by gender, stage of liver disease, GT HCV and history of prior therapy documented an advantage for genotype-specific vs. “new” pangenotypic options in men and patients with cirrhosis (Fig. 3).
Fig. 2
Treatment effectiveness in patients treated with genotype-specific and pangenotypic regimens (excluding SOF + RBV regimen) according to kidney function in per protocol (PP) analysis; HD – hemodialysis, Ktx – kidney transplantation
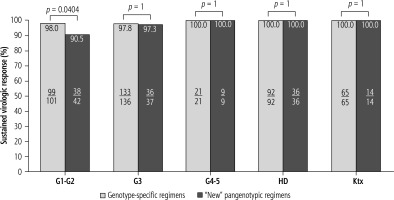
Fig. 3
Treatment effectiveness in patients treated with genotype-specific and pangenotypic regimens (excluding SOF + RBV regimen) according to gender, genotype (GT), liver fibrosis stage and history of previous treatment in per protocol (PP) analysis; SOF – sofosbuvir, RBV – ribavirin, F – fibrosis stage, T – treatment
Among patients treated with proton-pump inhibitors, statins, and anticoagulants, no significant differences in the virologic response or the percentage of adverse effects were noted. Similar data can be seen when analyzing both groups concerning comorbidities: arterial hypertension, diabetes, autoimmunological disorders, lipid disorders, and tumors other than HCC.
Table 5 shows 13 patients, who did not achieve SVR 12. The group consisted of 3 women and 10 men.
Table 5
Characteristics of 13 virologic failures with genotype-specific and pangenotypic regimens
[i] AH – arterial hypertension, ASV – asunaprevir, CP – Child-Pugh scale, CVD – cardiovascular disease, DCV – daclatasvir, DM – diabetes mellitus, DSV – dasabuvir, EBR – elbasvir, EOT – end of treatment, F – fibrosis, GLE – glecaprevir, GT – genotype, GZR – grazoprevir, HCC – hepatocellular carcinoma, KTx – kidney transplantation, M – male, MPGN – membranoproliferative glomerulonephritis, OBV – ombitasvir, PIB – pibrentasvir, PTV/r – paritaprevir, RBV – ribavirin, SOF – sofosbuvir, TD – target detected, TND – target not detected, VEL – velpatasvir, W – women
In 8 of these cases, liver cirrhosis had been confirmed before treatment started, while 2 patients had no data on the level of fibrosis. Genotype 3 HCV was identified in 4 patients.
When comparing patients who responded to antiviral treatment and virologic non-responders, several factors can be identified, which can potentially be related to the lack of response to treatment. These are male gender, older age, overweight, liver cirrhosis, history of decompensation, GT3, and hypoalbuminemia. However, only liver cirrhosis reached statistical significance.
Safety
In both groups, genotype-specific and pangenotypic, the course of treatment by the regimen was comparable (92.5% and 90.9%). The situation was similar when the treatment was terminated or modified.
Changes to the therapeutic regimen usually were related to the decrease of dosage or discontinuation of RBV due to anemia. One of the patients, due to black vomit, itchy rash, and nightmares, discontinued the treatment for 10 days. The treatment was then continued and the patient ultimately achieved SVR12. A different patient had the treatment extended from 8 to 12 weeks.
In the genotype-specific group, at least one adverse effect was reported significantly more frequently as compared to the pangenotypic group (39.5% vs. 25.5%, p = 0.0014). The most common adverse effects reported were weakness and fatigue, as well as anemia, all documented at a significantly higher rate in patients receiving genotype-specific regimens. These symptoms occurred also noticeably more often in patients who were administered RBV (Table 6).
Table 6
Safety of antiviral therapy of infected patients
Ten patients with decompensated liver cirrhosis – class B according to the CP classification – received a regimen with protease inhibitor. Liver decompensation progressed in three of these patients, and in two cases the treatment had to be discontinued. They were all treated with OBV/PTV/r ± DSV ± RBV.
In total, during the study period, 14 patients died, 7 in each of the two groups. None of the cases were related to the antiviral treatment according to the researchers.
Discussion
The development of noninvasive methods of liver fibrosis level assessment and access to highly effective and safe DAA made it possible to modify the approach to the treatment of the most difficult patients with chronic HCV infection [16]. Such patients included those with advanced kidney failure, hemodialyzed, and patients after kidney transplantation [17, 18].
The SVR rate in patients treated with IFN and RBV was only slightly above 40%; in 30% it was necessary to terminate the treatment for various reasons, which were often severe adverse effects, and the lack of prospects for a safe transplant more often than not determined the life of patients with CKD [19].
The first IFN-free treatment with SOF in 2014 still did not overcome the barrier for patients with GFR < 30 ml/min/1.73 m2. The drug was not registered for this patient population due to the risk of accumulation and toxicity of its metabolites in those with kidney failure of stage 4 or greater [20]. Additionally, the regimen SOF + RBV, despite its improved virological response percentage, still remained sub-optimal. Of the 17 patients treated with SOF + RBV in our population, 82% achieved SVR, which is in line with the results of other studies [21]. Three failures were in patients with GT3 infection, another two patients also had cirrhosis and one was after a kidney transplantation. Similar conclusions were presented by Feld et al. in the summary of the TARGET Study [22]. Patients with GT3 infection and cirrhosis achieved 58% SVR or even responded in a lower rate (42%) if they had a failed antiviral treatment in their history. In the following years, studies summarized in the Fabrizi et al. meta-analysis (overview of 6 studies with 69 patients) and another one by Li et al. (21 studies, 717 patients) in the 4th and 5th stage of CKD, confirm their stable function during and after treatment with SOF [9, 23].
Hence the newest recommendations from EASL and AASLD for patients with impaired kidney function suggest treatment following general guidelines, without the need to adjust the DAA dosage regardless of the level of kidney failure [14, 15]. The only remark refers to RBV if the antiviral regimen includes it in the treatment.
In both recommendations, a treatment alternative can be found for patients with GFR < 30 ml/min/1.73 m2, with end-stage renal failure and hemodialyzed. These include both genotype-specific regimens (GZR and EBR) and pangenotypic (GLE and PIB). Such choices can be considered after the analysis of the ERCHIVES Study data published by Butt et al. [24]. The lowering of GFR > 10 ml/min/1.73 m2 in patients with stage 4-5 CKD was decidedly more often noted in those treated with LDV/SOF than LDV/SOF + RBV, or OBV/PTV/r + DSV (6.5% vs. 3.3% vs. 1.8% respectively). In our study, the most often chosen genotype-specific regimen for hemodialyzed patients and those with CHKD G4-G5 infected with GT1was GZR/EBR ± RBV. The response to treatment was excellent, with only 1 in 143 patients not achieving SVR. This was a patient with liver cirrhosis and HCC. Equally excellent results in patients with HCV GT1 infection and stage 4-5 CKD were presented by Roth et al. in the C-SURFER study [25]. Ignoring the patients who discontinued the treatment due to reasons other than virological failure, SVR was achieved by 99% of the treated individuals. Alric et al., confirming the 97% success rate of the treatment, referred to its safety and drug interactions [26]. Similarly as in our observation, in most cases, patients did not require any adjustments in the dosage of concomitant drugs. The necessity of starting renal replacement therapy, the discontinuation of antiviral treatment, or the patient’s death was in more cases related to a comorbidity than the selected treatment course. This is confirmed by a temporary, at EOT, improvement in renal function in 12 out of 32 patients with CKD stage 3 presented by Reddy et al., of whom 50% maintained an improved GFR > 60 ml/min/1.73 m2 at follow-up week 12, while the remaining patients’ parameters decreased to their initial values [27].
In the EpiTer-2 study, the LDV/SOF ± RBV regimen was significantly more often chosen for patients with GFR > 30 ml/min/1.73 m2. This treatment was administered to 135 patients (31.5%), including 42 (31.1%) after kidney transplantation. All of the patients available for a follow-up assessment achieved SVR after 12 weeks. Stable graft functioning was reported in 31 patients after transplantation at EOT and for consecutive months [28]. In fact, Morales et al. reported the worsening of graft function in 28.1% (n = 9) of patients treated with LDV/SOF [29]. However, this was a temporary state, and in summary, all patients returned to the initial creatinine levels after the EOT. In addition, in patients treated with calcineurin inhibitors, fluctuations in tacrolimus or cyclosporin levels are an additional factor influencing renal function. These circumstances were not encountered by Okubo et al., who managed to confirm stable renal function in 132 patients with CKD stage 3 before treatment, at EOT, and 12 weeks after completing the treatment with LDV/SOF, whose eGFR was 51%, 52.2%, and 52.7% respectively [30].
Bringing the genotype-specific treatment topic to a close, it is necessary to mention the, now historic, regimen of OBV/PTV/r + DSV ± RBV, which was administered to 31.5% of patients (n = 135). Although the virological failure rate was the highest in comparison to all other genotype-specific treatment options (23.1% vs. 15.4%), 98% still achieved SVR. These results are analogous to those presented by other realworld researchers, such as Sanai et al. – 100% SVR PP, Sperl et al. – also 100% SVR, or Lawitz et al. in RUBY-I and RUBY-II – 94% and 96% SVR, or in the meta-analysis by Pogorzelska et al. [31–34].
It is worth underlining that our patients with more advanced kidney failure (G4-G5), hemodialyzed and after kidney transplantation responded to the antiviral treatment in 100% of cases. These data differ from those presented by Wiegand et al., where SVR was achieved by 89% and 92% of patients with GFR > 15-30 ml/min/1.73 m2 and 0-15/HD (hemodialyzed) respectively [35].
Pangenotypic regimens of GLE/PIB and SOF/VEL with or without RBV have been available in Poland since 2018. It is worth noting that although the current analysis showed no overall differences in SVR rates in patients treated with these options compared to genotype-specific regimens, we documented their significantly lower effectiveness in men and patients with cirrhosis. It is difficult to clearly comment on these findings and refer to other studies due to the different effectiveness reported for individual DAA regimens, both in clinical trials and real-world studies, depending on the baseline characteristics of the analyzed populations [36].
In our study, the GLE/PIB regimen was administered to half of the patients. This was the most popular treatment offered to hemodialyzed patients. Neither glecaprevir nor pibrentasvir is secreted through the kidneys, which made this pangenotypic treatment preferred from the start in patients with renal failure. Only one of our patients, who did not achieve SVR, had to discontinue the treatment after two weeks due to adverse effects. Gane et al. in NEJM presented equally excellent results with an SVR rate of 98% from a multi-center study of stage 3 EXPEDITION-4 [37]. The study assessed the effectiveness and safety of 12-week treatment with GLE/PIB in patients with stage 4 and 5 renal failure, mostly hemodialyzed. The most frequent adverse effect in these patients was itchiness, which differed from the adverse effects obtained in our study. It needs to be remembered, however, that in patients with calcium-phosphate homeostasis disorders, observed in secondary hyperparathyroidism resulting from renal failure, itchiness is a very common phenomenon. At the same time, a drug interaction strengthening this effect cannot be excluded. In a modified study protocol, EXPEDITION-5, the authors assessed 8, 12, and 16-week treatment with GLE/PIB in patients with stage 3b,4, and 5 CKD. The duration of treatment was dependent on GT, liver fibrosis, and a history of non-responsiveness to treatment. Excluding the patients who discontinued the treatment (n = 2) or were not available for follow-up observation (n = 1), an excellent result of 100% SVR was achieved with no adverse effects related to DAA. Of the 24 patients with CKD 3b or 4, none had any significant changes registered in average eGFR since the beginning of the study, through their EOT, and up to 4 weeks after the treatment ended [38].
In the analysis of the 13 non-responders in our cohort, 4 (30.8%) were treated with SOF/VEL ± RBV. All these patients had liver cirrhosis, with one patient having decompensation of the liver and another one being infected with GT3, and another 2 patients, due to their alcohol issues and mental disorders, were suspected not to be taking their medications regularly. Looking at the entire SOF/VEL ± RBV subgroup as a whole, the SVR was 93.3%. It needs to be noted that this regimen was most often chosen for patients with GFR > 30 ml/min/1.73 m2. Hemodialyzed patients, or those treated with peritoneal dialysis, received SOF/VEL in a multi-center study of stage 2 presented by Borgia et al. [39]. Regardless of the genotype, history of treatment failure, and fibrosis stage, an SVR was achieved by 95% of patients. Additionally, in the majority of cases due to comorbidities, patients were administered many other drug groups potentially interacting with the DAA, which in turn could reduce the antiviral effectiveness, or increase the toxicity of the other drugs. While describing the adverse effects, the authors have, however, excluded their relation with the DAA [38]. In a collective study summary by Fabrizi et al. on SOF/VEL the drop-out rate due to adverse effects in advanced renal failure was < 5% and in the majority of studies no one discontinued therapy [9].
Adverse effects reported in our study do not differ from the majority of reports. The most often reported ones are fatigue/weakness and anemia. These adverse effects were noticeably more often reported by patients treated with RBV. Adverse effects leading to treatment discontinuation and death were more frequent in pangenotypic treatments in comparison to the genotype-specific arm (0.2% vs. 3% and 1.6% vs. 4.2% respectively), although attention needs to be drawn to 3 out of the 15 patients with liver cirrhosis, a history of liver decompensation or qualified to B or C on the CP scale treated with genotype-specific regimens.
In all cases, researchers excluded the relation between DAA and decompensation or death of the patients. However, 2 patients treated with a protease inhibitor (OBV/PTV/r ± DSV) had, at the time of starting treatment, liver cirrhosis, CP B, and both discontinued treatment in the 4th week due to decompensation. They both had contraindications for liver transplantation.
One may ask whether the antiviral treatment should have been discontinued in these patients. Or should another therapeutic regimen have been suggested? At least 7 patients with decompensated liver cirrhosis had strong contraindications for a liver transplantation/simultaneous liver and kidney transplantation. Looking at data by Gentile et al. from the Italian Liver Network Activity (LINA) cohort, antiviral treatment could have been the only chance to stabilize liver function and extend survivability [40]. Of the 89 patients with CP B liver cirrhosis, throughout the first month of treatment and during the last observation 45.5% and 61.8% patients respectively improved their liver function, getting qualified as class A CP. Only 4 (4.5%) patients had deterioration of their liver function (CP B → CP C), and 3 (3.4%) experienced at least one decompensation episode during treatment.
Of the 2 of our patients with CP C liver cirrhosis treated with LDV/SOF ± RBV, one died in the 20th week of treatment due to a hemorrhage from gastric varices. Tahata et al. reported nearly 50% of CP C liver cirrhosis decompensation episodes during DAA treatment [41]. In the case of patients with CP B, decompensation and subsequent hospitalization occurred in 12.1% of cases.
The main limitation of the study is the non-homogeneous observation group, which reduces the total number of individual subgroups and makes drawing definite conclusions difficult.
Studies conducted in real-world conditions relate to therapeutic processes introduced in real clinical environments and they should be understood as such. On the other hand, due to the difficulty in avoiding researcher subjectivity, related to the choice of the treatment regimen, its modifications, adverse effect reporting as well as a differing outlook on the issue, there is a potential risk of an incomplete set of coherent data in the complex summary.
Conclusions
Both regimens, the genotype-specific and pangenotypic, are equally effective and safe in patients with renal failure. The stage of kidney failure, renal replacement therapy, and transplants do not influence the antiviral response. The majority of failures of the antiviral treatment are a result of the selection of the “old” pangenotypic regimen of SOF + RBV, premature discontinuation of treatment due to adverse effects, and the potential lack of cooperation from the patient.






