Introduction
Lung adenocarcinoma (LUAD) is a non-small cell lung cancer that originates from the inner wall cells of the lungs, representing 40% of all lung cancer cases [1-3]. Currently, therapeutic options for LUAD include surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy [4, 5]. Most targeted therapy drugs act on cancer-associated specific proteins, exerting various anti-cancer effects while causing relatively little damage to normal cells [6-9]. Similarly, the pharmacological mechanism of some drugs in immune therapy for LUAD is achieved by blocking the function of specific marker proteins, ultimately achieving therapeutic efficacy [10]. Targeted therapy and immune therapy targets are often proteins that are abnormally expressed or have abnormal structures in cancer cells, such as EGFR, ALK, ROS1, PD-L1, etc. [11]. STK11, as a classic biomarker, has a certain value for prognostic prediction of lung cancer patients [12].
One of the most frequently altered genes in LUAD is STK11 [13]. The STK11 gene produces a protein that functions as a tumor suppressor and is essential for regulating the development, proliferation, and differentiation of tumor cells [14, 15]. Therefore, in order to create more potent targeted therapeutics, it is essential to comprehend the molecular basis of the STK11 mutation in LUAD. Similar to the common mutation of another gene, EGFR, in LUAD, EGFR inhibitors (such as gefitinib, erlotinib, and osimertinib) can block the function of the EGFR protein, and these drugs have shown good therapeutic effects in EGFR-mutated patients [16-18]. Serine/threonine kinase (BRAF) is another gene that can mutate and promote cancer growth in LUAD patients. BRAF inhibitors (such as dabrafenib and trametinib) can target the mutated BRAF protein and help slow tumor growth [19-21]. Despite the fact that there are no reports of STK11 inhibitors being used successfully right now, recent studies have shown that patients with STK11 mutation may benefit from targeted therapies, such as targeting the mTOR pathway [22] or Hippo pathway [23]. Therefore, exploring the regulatory pathways of STK11 has enormous potential for development in the treatment of LUAD.
CD4+ T cells (helper T cells) are immune cells that are critical in adaptive immune responses [24, 25]. CD4+ T cells regulate other cells in the immune system, and are essential for defending against intracellular pathogens (such as viruses and certain bacteria) and activating other immune cells (such as B cells and CD8+ T cells) [26-28]. CD4+ T cells have various subtypes, including Th1, Th2, Th17, Treg, and Tfh cells, each with unique functions and cytokine profiles [29, 30]. Th1 cells are involved in defense against intracellular pathogens [31], while Th2 cells play a role in the response to extracellular parasites, bacteria, allergens, and toxins [32-34]. Th17 cells are crucial for defense against bacteria and fungi [35], and Treg cells assist in controlling immune responses to prevent autoimmunity [36]. Defective CD4+ T cell function can lead to immune deficiency diseases, such as HIV/AIDS [37, 38], while overactivation of CD4+ T cells can lead to autoimmune diseases such as multiple sclerosis and lupus [39, 40]. CD4+ T cells have a complex role in immune responses in cancer. First, CD4+ T cells promote anti- tumor immune responses by activating other immune cells and helping to kill cancer cells [41, 42]. Second, CD4+ T cells can actually drive cancer cell proliferation by providing growth factors and cytokines that stimulate cancer cell growth and division [43]. The current research focus is on finding a balance point between these pro-tumor and anti-tumor effects of CD4+ T cells and developing new immunotherapies to enhance anti-tumor responses and facilitate prognosis of LUAD patients [44, 45]. The complex interactions between CD4+ T cells and cancer cells in LUAD, and how best to target this relationship for therapeutic benefit, remain to be explored.
This study mainly investigated the impact of STK11 mutation in LUAD on the shape of LUAD cells and CD4+ T cells by in vitro cell experiments. Finally, our study showed that STK11 mutation in LUAD cells could promote LUAD cell proliferation and migration while repressing the activity of CD4+ T cells.
Material and methods
Clinical samples
Clinical information of patients who had not received any treatment was collected from Wenzhou People’s Hospital between March 2022 and May 2023. By genetic detection, tumor tissues with wild-type STK11 (n = 15) and tumor tissues with mutant-type STK11 (n = 15) were found. All patients included in the study signed an informed consent form, and the experimental procedures involving clinical samples and information were approved by the Ethics Committee of Wenzhou People’s Hospital.
Cell culture
A549, H460, and H2030 cells carrying STK11 mutation and the Calu-6 cells without STK11 mutation were accessed from ATCC (USA). The H460 and H2030 cells were cultured in RPMI-1640 complete medium with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (P-S), the A549 cells were cultured in F-12K complete medium with 10% FBS and 1% P-S, and Calu-6 cells were maintained in MEM complete medium with 10% FBS and 1% P-S. According to the STK11 gene sequence, corresponding sgRNA sequences were designed and inserted into the eSpCas9-2A-GFP plasmid (GenScript, China). Lipofectamine 3000 (Thermo Fisher, USA) was utilized to transfect the plasmids into A549 and Calu-6 cells to construct A549STK11 RES and Calu6STK11 KD cell models, which were respectively cultured in F-12K and MEM complete medium with 10% FBS and 1% P-S at 37°C and 5% CO2.
Human PBMC cells were purchased from Zhejiang MeisenCTCC. CD4+ T cells were extracted from PBMC using a negative isolation method with the Human CD4+ T Cell Isolation Kit (Thermo Fisher, USA) and stimulated with 2.5 µg/ml CD3 and 2 µg/ml CD28 antibodies (Gibco, USA) for 48 h to induce activation. The constructed Calu6STK11 KD and Calu6STK11 WT cells in a 1 : 10 ratio were co-cultured with activated CD4+ T cells in RPMI-1640 medium with 10% FBS and 1% P-S at routine condition.
qRT-PCR
A cell suspension of A549 (A549STK11 RES and A549STK11 MUT) and Calu-6 (Calu6STK11 KD and Calu6STK11 WT) was collected. Total RNA was extracted from cells with TRIzon Reagent (Cwbio, China), and reverse transcription was done with the Hifair II 1st Strand cDNA Synthesis Kit (Yeasen, China). Using cDNA as the template, qRT-PCR was completed using UltraSYBR Mixture (Cwbio, China) and the ABI7500 system (Thermo Fisher Scientific, USA). The total cDNA was obtained after multiple high-temperature denaturation (95°C), low-temperature annealing (60°C), and elongation. With β-actin as an internal reference gene, the 2-ΔΔCt method was used to determine the relative expression level of STK11. Experiments were repeated three times for each group. The primer sequences are shown in Table 1.
Cell Counting Kit-8 assay
A549 (A549STK11 RES and A549STK11 MUT) and Calu-6 (Calu6STK11 KD and Calu6STK11 WT) cell viabilities were measured with cell counting kit-8 (CCK-8; EZB-CK8, China). A 100 µl cell suspension (5 × 103 cells) was added to each well of a 96-well plate for culture in a cell culture incubator for 0 h, 24 h, 48 h, 72 h and 96 h. The medium was replaced with serum-free medium, and each well was supplemented with 10 µl of CCK-8 solution, followed by 4 h of incubation. Finally, the OD value was assessed at 450 nm by a microplate reader. Each group was tested in triplicate.
Colony formation assay
The A549 (A549STK11 RES and A549STK11 MUT) and Calu-6 (Calu6STK11 KD and Calu6STK11 WT) cell suspension was diluted and seeded into a 6-well plate, with 500 cells per well, and incubated in a 37°C, 5% CO2 incubator for 14 days. After rinsing twice with PBS, colonies were fixed with 75% ethanol for 15 min, stained with crystal violet, and colonies with more than 50 cells were scored as viable colonies. The images of colonies were captured with a digital camera. The experiment was done three times.
EDU assay
A549 (A549STK11 RES and A549STK11 MUT) and Calu-6 (Calu6STK11 KD and Calu6STK11 WT) cells were seeded (5 × 105 cells/well) in a 96-well plate and cultured until adherent. The EDU cell proliferation detection kit (Ribobio, China) was used. EDU solution was diluted to a certain ratio with complete medium, and 100 µl was added to each well of the 96-well plate. After washing with PBS, cells were fixed with 4% paraformaldehyde for 30 min, stained with glycine for 5 min, and then incubated with 0.5% Triton X-100 for 5 min. After staining with Apollo for 30 min, cells were incubated with 0.5% Triton X-100 again for 5 min. Then, 100 µl of 1X Hoechst 33342 reaction solution was added to each well for DNA staining, and cells were observed and photographed under a fluorescence microscope (Leica DM 4000B, Germany).
Cytotoxicity assay
The cytotoxic effect of CD4+ T cells on tumor cells was assayed with an lactate dehydrogenase (LDH) cytotoxicity assay kit (Yeasen, China). Effector cells and target cells were plated in a 96-well plate at a ratio of 10 : 1, and different control groups were set up according to the instructions and experimental grouping. After incubation in a cell culture incubator for 4 h, the supernatant of each well was harvested and transferred to a new 96-well plate. Reaction substrate was added and incubated for 30 min, and the optical density value was assessed at 490 nm. Each group was tested in triplicate.
Transwell assay
Transwell chambers (LABSELECT, China) were placed in a 24-well plate. CD4+ T cells were plated into the upper chamber (1 × 105 cells/ml), and the supernatant from the treated tumor cells or RPMI-1640 was added to lower chamber. Chambers were incubated in a 37°C, 5% CO2 incubator for 4 h, and migrated cells were collected and counted using a cell counting plate. Each group was tested in triplicate. The migration index was calculated as: the number of migrated cells in the experimental group/that in blank group (without chemotactic factors).
ELISA
We detected the content of the cytokines interferon γ(IFN-γ), interleukin (IL)-2, and tumor necrosis factor α (TNF-α) in the supernatant of tumor cells co-cultured with CD4+ T cells according to the instructions of the ELISA kit manufacturer. Specifically, the supernatant from the co-culture system was collected using the Human IFN-γELISA Kit (ab174443), Human IL-2 ELISA Kit (ab270883), and Human TNF-α ELISA Kit (ab181421) for detecting IFN-γ, IL-2, and TNF-α. All the above test kits were purchased from Abcam (UK).
Immunohistochemistry (IHC)
The collected patient tumor tissue was dehydrated and embedded in paraffin to prepare sections. After baking, the sections were immersed in xylene I and II for 20 min each to remove paraffin, and then hydrated in 100%, 95%, 85%, and 75% ethanol for 5 min each. Tissue was subjected to antigen retrieval in EDTA solution, followed by sealing of endogenous peroxidase with hydrogen peroxide enzyme-blocking solution. After washing with PBS twice for 5 min each time, the tissue was incubated with normal goat serum homologated with secondary antibody at 37°C for 30 min. Anti-STK11 (Proteintech, China) primary antibody was added, and the tissue was placed overnight in a 4°C refrigerator. The next day, the sections were washed with PBS twice for 5 min each time, incubated with HRP-labeled goat anti-rabbit secondary antibody (Beyotime, China) for 30 min, and stained with DAB. Staining results were observed under an optical microscope, and ten fields (400×) containing positively stained cells were randomly selected for analysis and the average value was recorded for each section.
Immunofluorescence (IF)
Paraffin sections were dewaxed and hydrated as described in step 2.10, followed by antigen retrieval and blocking with normal goat serum. Sections were then incubated with CD4 (Proteintech, China) primary antibody and placed in a refrigerator at 4°C overnight. Following rinses with PBS, sections were incubated with a fluorescently labeled secondary antibody (Alexa Fluor 488) (Bioss, China) in the dark at 37°C for 30 min. DAPI was added for 5 min of staining in the dark. After rinsing with PBS, sections were sealed with an anti-fluorescence quenching sealing agent and observed under a fluorescence microscope.
Data analysis
Data were presented as mean values ± standard deviation (mean ±SD). Based on GraphPad Prism 8.0 software, all data were statistically evaluated with at least three biological replicates for every experiment. For comparisons between two individuals, the non-parametric Mann-Whitney test was employed, and the non-parametric Kruskal-Wallis test was used for comparisons between several individuals. P < 0.05 was regarded as statistically significant.
Results
STK11 mutation drives LUAD progression
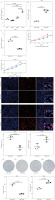
To further validate expression of STK11 in different LUAD cells, we measured STK11 expression in A549, H460, H2030 cells carrying STK11 mutation and Calu-6 cells without STK11 mutation. Compared with Calu-6 cells, STK11 expression in A549, H460, and H2030 cells was significantly lower (p < 0.0001) (Fig. 1A), indicating a significant loss of STK11 expression in LUAD cells with STK11 mutation. We then constructed A549STK11 RES and Calu-6STK11 KD cell models to investigate the effect of STK11 mutation on LUAD. qRT-PCR revealed that mRNA levels of STK11 were significantly lower in A549STK11 MUT cells than in A549STK11 RES cells (p = 0.0052) (Fig. 1B). qRT-PCR revealed that mRNA levels of STK11 were significantly lower in Calu-6STK11 KD cells than in Calu-6STK11 WT cells (p = 0.0046) (Fig. 1C). CCK-8 assays showed that the loss of the STK11 gene promoted the OD value of A549STK11 MUT (p = 0.0065) and Calu-6STK11 KD (p = 0.0104) cells (Fig. 1D, E). Furthermore, the EDU assays indicated that the proliferation ability of A549STK11 MUT (p = 0.0012) and Calu-6STK11 KD (p = 0.0099) cells was significantly enhanced (Fig. 1F, G). The colony formation assays also indicated that the proliferation ability of A549STK11 MUT (p = 0.0042) and Calu-6STK11 KD (p = 0.0224) cells was significantly enhanced (Fig. 1F, G). These results suggested that STK11 mutation in LUAD cells led to a decrease in STK11 expression and promoted the malignant progression of LUAD.
Fig. 1
STK11 mutation promotes the progression of LUAD. A) qRT-PCR verification of the expression levels of STK11 in different LUAD cells: A549, H460, H2030 and Calu-6 (p < 0.0001). B) qRT-PCR detection of transfection efficiency in A549STK11 RES and A549STK11 MUT cells (p = 0.0052). C) qRT-PCR detection of transfection efficiency in Calu6STK11 KD and Calu6STK11 WT cells (p = 0.0046). D) CCK-8 detection OD value (450 nm) of A549STK11 RES and A549STK11 MUT cells (p = 0.0065). E) CCK-8 detection OD value (450 nm) of Calu6STK11 KD and Calu6STK11 WT cells (p = 0.0104) F) EDU experiment to detect proliferation of A549STK11 RES and A549STK11 MUT cells (p = 0.0012) and Calu6STK11 KD and Calu6STK11 WT cells (p = 0.0099) G) Colony formation experiment to detect proliferation of A549STK11 RES and A549STK11 MUT cells (p = 0.0042) and Calu6STK11 KD and Calu6STK11 WT cells (p = 0.0224). Data are presented as mean values ± standard deviation (mean ±SD). P < 0.05 was regarded as statistically significant. Each set of experiments was repeated three times
STK11 mutation affects the CD4+ T cell-mediated killing effect on LUAD cells
Studies have shown that LUAD patients with STK11 mutation have less immune cell infiltration, such as B cells, CD8+ T cells, CD4+ T cells, macrophages, neutrophils, etc. [46]. To investigate the effect of CD4+ T cells on LUAD cells with STK11 mutation, we isolated CD4+ T cells from human PBMC cells, activated them, and co-cultured them with STK11 mutant and wild-type LUAD cell lines (Calu-6STK11 KD and Calu-6STK11 WT). Cell toxicity and chemotaxis assays assessed killing ability and chemotaxis of CD4+ T cells against LUAD cells. Compared with Calu-6STK11 WT cells, the cytotoxicity of CD4+ T cells against Calu-6STK11 KD cells was significantly lower (p = 0.0192), and their chemotaxis ability was also significantly lower (p = 0.0085) (Fig. 2A, B). ELISA analysis of the cytokine expression levels in the supernatant of CD4+ T cells co-cultured with Calu-6 cells revealed a substantial decrease in IFN-γ(p = 0.0015), IL-2 (p = 0.0021), and TNF-α (p = 0.015) levels (Fig. 2C-E). Thus, STK11 mutation substantially reduced the killing ability of CD4+ T cells against LUAD cells.
Fig. 2
The effect of LUAD STK11 mutation on the activation and killing effect of CD4+ T cells. A) Cytotoxicity of CD4+ T cells in co-culture of CD4+ T cells and tumor cells (p = 0.0192). B) Chemotaxis of CD4+ T cells in co-culture of CD4+ T cells and tumor cells (p = 0.0085). C-E) ELISA detection of levels of cytokines IFN-γ (p = 0.0015), IL-2 (p = 0.0021) and TNF-α (p = 0.015) in co-culture of CD4+ T cells and tumor cells. Data are presented as mean values ± standard deviation (mean ±SD). P < 0.05 was regarded as statistically significant. Each set of experiments was repeated three times

STK11 mutation leads to decreased immune cell infiltration in LUAD patients’ tumor tissues
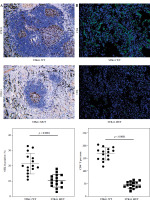
To delineate the modulatory role of STK11 mutation in the tumor microenvironment of LUAD, we evaluated the levels of immune cell infiltration in clinical tissue samples from patients with wild-type STK11 and mutant LUAD. By performing IHC analysis to assess STK11 protein expression in different patient tumor tissues, we found that STK11 protein expression in tumor tissues of STK11 mutant patients was substantially lower than that in tumor tissues of wild-type STK11 patients (p < 0.0001) (Fig. 3A). IF analysis of CD4+ T cell infiltration levels in tumor tissues of STK11 wild-type and mutant LUAD patients showed that CD4+ T cell content was significantly lower in tumor tissues of STK11 mutant patients compared to that of STK11 wild-type patients (p < 0.0001) (Fig. 3B). The analysis of clinical tissue samples from LUAD patients revealed that STK11 mutation led to a decrease in immune cell infiltration in tumor tissues, confirming the important modulatory role of STK11 mutation in the tumor microenvironment of LUAD.
Fig. 3
Correlation analysis of STK11 and CD4 expression in clinical samples of LUAD. A) IHC detection of STK11 expression in tissues of wild-type and mutant LUAD patients (feature image) (p < 0.0001). B) IF detection of CD4+ T cell infiltration levels in tissues of wild-type and mutant LUAD patients (feature image) (p < 0.0001). Data are presented as mean values ± standard deviation (mean ±SD). P < 0.05 was regarded as statistically significant. Each set of experiments was repeated 15 times
Discussion
Lung adenocarcinoma has a high mortality rate [47]. STK11 mutation is common in LUAD and is associated with poor prognosis [48]. This study explored the potential mechanism of the impact of STK11 mutation on LUAD cells and tissues.
STK11 mutation is associated with lung cancer [49], non-small cell lung cancer [50], hereditary pancreatic cancer [51], breast cancer [52], and LUAD [53]. It is worth noting that LUAD can be caused by various genetic and environmental factors, and STK11 gene mutations are the commonest [13, 54]. Mutations in STK11 can lead to uncontrolled cell growth and tumor formation [55, 56]. We first investigated the effect of STK11 gene mutations on tumor progression through gene knockout experiments and found that STK11 mutation promotes the progression of A549 and Calu-6 cells, consistent with a report published in APMIS in 2022 [55]. There are currently no reports on whether STK11 mutation has a beneficial or detrimental effect on the cell activity of CD4+ T cells. This study, through successive experiments, first demonstrated that STK11 mutation inhibited the cell activity of CD4+ T cells in a co-culture system with Calu-6 cells.
Immune infiltration refers to the process by which immune cell migrate from the blood to tissues or organs to resist foreign invaders (such as pathogens or cancer cells). It plays a pivotal role in the immune system’s capacity to identify and react to foreign antigens, as well as to maintain tissue homeostasis and prevent the development of autoimmune diseases [57-60]. Previous studies have shown through in vivo and in vitro experiments that STK11 mutation significantly reduces infiltration levels of NK cells, and inhibits their activity and chemotaxis, and killing of LUAD cells, thereby promoting the progression of LUAD [55]. In addition, a study published in Frontiers in Oncology in 2020 showed that STK11 gene copy number is connected with immune cell infiltration, and the immune cell infiltration levels of LUAD patients with STK11 mutation are much lower than those of the wild type [13]. Subsequently, we investigated the impact of STK11 mutation on immune infiltration levels based on the collected clinical samples and found that STK11 mutation significantly reduced the immune infiltration levels of CD4+ T cells in LUAD tissues, which is consistent with previous research findings.
Based on the above discussion, we concluded that STK11 mutation in LUAD tissues led to reduced infiltration and activation of CD4+ T cells, thereby promoting tumor growth by producing an immune-suppressive microenvironment. The reduced activity of CD4+ T cells observed in tumors with STK11 mutation may represent an immune evasion mechanism. This study revealed a mechanism by which STK11 mutation modulated CD4+ T cells and promoted tumor progression, providing fresh insights into role of STK11 mutation in LUAD and their effects on the immune system. Future research needs to explore likely mechanisms by which STK11 mutation affects immune cell activity and develop effective treatments for STK11 mutation in LUAD.


