Purpose
Stereotactic body radiation therapy (SBRT) is a safe and effective treatment option for patients with early-stage non-small cell lung cancer (NSCLC). While 5-year local tumor control rates after SBRT are greater than 90% [1, 2], isolated primary tumor site progression can occur in the absence of metastatic disease due to biological radio-resistance [3], insufficient doses delivered to tumor target [4], or potential marginal misses during treatment delivery due to inaccurate image guidance delivery systems [5]. Secondary treatment options for isolated primary tumor site progression after SBRT include salvage lung resection, re-irradiation with external beam radiation therapy (EBRT), or percutaneous ablative procedures (cryotherapy, microwave ablation, radio-frequency ablation) [6, 7]. A more novel approach is percutaneous high-dose-rate (HDR) brachyablation that is a minimally invasive treatment involving implantation of temporary catheters into lung tumors to deliver high doses of radiotherapy in a single fraction with minimal exposure of previously irradiated tissues [8, 9].
Since 2005, a limited number of case series have been reported in the literature demonstrating the safety and efficacy of percutaneous HDR brachyablation for lung tumors [8, 10-13]. Our institution has been delivering this treatment since 2015, and published our initial outcomes in 2021 for 25 patients with 37 pulmonary tumors treated [9]. In that report, the 2-year local control rate with upfront HDR brachyablation of lung tumors was 96%, with no patients developing a major complication. We did observe one patient with radiographic evidence of minor pulmonary hemorrhage, and four patients required a small-caliber chest tube that was removed within 24 hours.
Herein, we summarized our first case of percutaneous HDR brachyablation to re-treat an early-stage NSCLC that progressed within the primary tumor site several years after initial SBRT.
Case presentation
The patient was a 74-year-old female with a remote history of breast cancer treated with mastectomy and adjuvant endocrine therapy; she did not receive any radiotherapy for her breast cancer. A chest X-ray obtained for chest pain nine years after definitive treatment for breast cancer demonstrated an incidentally detected new density in the right lower lobe (RLL). She had a 40 pack-year history of smoking and a diagnosis of chronic obstructive lung disease. A subsequent chest CT revealed a 3.3 × 3.1 × 2.8 cm mass in the anteromedial basal segment of the RLL, and a CT-guided biopsy confirmed primary squamous cell carcinoma of lung origin without any epidermal growth factor receptor mutations. A [18F]-2-fluoro-2-deoxy-D-glucose (FDG)-positron emission tomography/computed tomography (PET/CT) staging demonstrated the RLL mass to be hypermetabolic (SUVmax 9.8) without abnormal uptake in the hilum, mediastinum, or other sites of the body. The resultant clinical stage was IB (cT2aN0M0) NSCLC, according to the AJCC cancer staging manual, 8th edition [1]. Pulmonary function testing identified a predicted forced expiratory volume in the first second (FEV1) of 45% and diffusing capacity of the lungs for carbon monoxide (DLCO) of 43%. The patient was deemed to be a poor surgical candidate by a board-certified thoracic surgeon, and was recommended definitive treatment with SBRT.
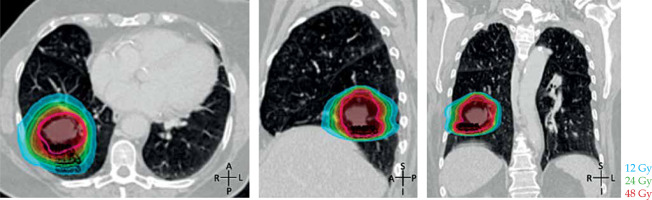
Stereotactic body radiation therapy treatment planning involved a vacuum mold immobilization device and four-dimensional CT (4D CT) simulation to capture all phases of the tumor movement throughout the breathing cycle, and utilized to contour internal gross target volume (iGTV). The iGTV was expanded isotopically by 5 mm to create the final planning treatment volume (87.8 cc). The prescription dose was 50 Gy in 4 daily fractions, planned using Monte Carlo (BrainLab.XVMC.X) calculations to generate a volumetric modulated arc therapy (VMAT) plan with three coplanar arcs using 6 MV photons (Figure 1). The final SBRT plan dose statistics confirmed a D95 of 48 Gy, Dmax of 134%, conformality index of 1.12, D2cm of 14.5 Gy, and R50 of 3.66. All treatments were delivered with an isocentric technique on a conventional linear accelerator after verification using daily on-board cone-beam CT images (Varian Medical Systems, Palo Alto, CA, USA; and BrainLAB, Feldkirchen, Germany).
Fig. 1
Initial stereotactic body radiation therapy (SBRT) treatment plan delivering 50 Gy in 4 daily fractions to a stage I nonsmall cell lung cancer of the right lower lobe
The patient underwent post-SBRT surveillance with serial chest CT scans at 3, 6, 10, and 13 months after treatment. At 20 months after completion of SBRT, a new 6 mm nodule was detected in the contralateral, left lower lobe (LLL). Biopsy confirmed a new primary squamous cell carcinoma of the lung. The metachronous second primary lung cancer in the LLL was successfully treated with a similar course of SBRT, and remains without evidence of progression or side effects at 5.1 years post-treatment.
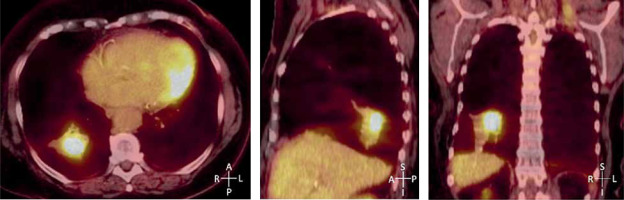
At approximately 3.8 years after SBRT for the initial NSCLC in the RLL, a FDG-PET/CT restaging scan demonstrated increased FDG uptake within the primary treated tumor measuring 4.9 × 3.4 cm with SUV 5.9 (Figure 2), suspicious for progression within the primary tumor site. A subsequent CT-guided biopsy confirmed the persistence of squamous cell carcinoma, confined to the primary tumor site at 4.3 years after initial SBRT delivery. Her case was discussed at a multi-disciplinary thoracic oncology program tumor board, and a repeat course of SBRT was not advised due to concerns about the risk of normal tissue injury. A recommendation was made for either percutaneous thermal ablation or percutaneous HDR brachyablation, with the latter preferred due to the relapsed tumor’s size measuring nearly 5 cm.
Salvage high-dose-rate brachyablation
Percutaneous HDR brachyablation treatment was performed as an outpatient procedure beginning with conscious sedation in an interventional radiology suite. The patient was medicated with midazolam, fentanyl, ketorolac, and ondansetron; prophylactic antibiotics were not administered. A diagnostic chest CT in the prone position was obtained to aid with target localization. The overlying skin was demarcated by an interventional radiologist, and 1% lidocaine was administered at the skin and underlying soft tissues followed by advancement of an Argon 17-gauge coaxial introducer needle to the parietal pleura, where 15 cc of 0.5% bupivacaine was injected to achieve pleural anesthesia (Argon Medical Devices, Plano, TX, USA). The introducer needle was advanced by the interventional radiologist under CT-guidance into and through the superior half of the targeted tumor (Figure 3A). A 4-Fr Flexi-guide brachytherapy catheter was then inserted into the coaxial needle, so that the tip of the catheter aligned with the tip of the outer coaxial needle. With similar technique, a second 17-gauge needle and 4-Fr catheter were placed into the inferior half of the targeted tumor to facilitate full treatment coverage, as had been determined necessary during pre-procedural image review. Marks and measurements were made on the external surface of the catheter to facilitate reproducible positioning for HDR brachyablation treatment planning and delivery. Both sets of needles and catheters were fixed in place. The above-described procedure was completed in 90 minutes, and the patient was then transported on a hospital bed to the radiation oncology department for further management by the brachytherapy team.
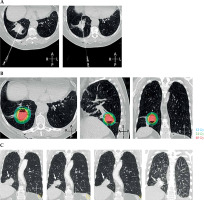
Fig. 3
Salvage high-dose-ratio (HDR) brachyablation. A) CT images in prone position demonstrating percutaneous introducer needles inserted into the inferior and superior portions of the targeted tumor. B) HDR treatment plan in prone position (irregular red line denote the contour of the relapsed tumor). C) Coronal CT scans at 6, 12, 24, and 33 months after salvage HDR brachyablation (white arrow – treated cancer)
Chest CT images that had been obtained in the interventional radiology suite were imported into the Oncentra® Brachy treatment planning system (Elekta Brachytherapy Solutions, Veenendaal, The Netherlands) to generate a treatment plan using an inverse treatment planning technique to deliver a single fraction of 24 Gy to the CTV (Figure 3B). Treatment planning parameters were according to the American Association of Physicists in Medicine (AAPM) Task Group 101 Report [14]. The final HDR brachyablation plan revealed a D90 of 28.1 Gy, D90% of 117.2, and D95 of 24.6 Gy. Organ at risk (OAR) dose constraints were a small bronchus limit of D0.035cm3 < 1330 cGy/fx, esophagus limit of D0.035cm3 < 1540 cGy/fx, and great vessel limit of D0.035cm3 < 3700 cGy/fx. Once the HDR brachyablation plan and quality assurance procedures were approved, the patient was relocated to a shielded brachytherapy HDR suite in the radiation oncology department to receive treatment through the two catheters. HDR brachyablation was delivered with iridium-192 from an HDR remote afterloader unit, and consisted of 48 dwell positions in its design. The duration of treatment delivery was 19.6 minutes. Following the delivery of HDR brachyablation, the patient was transported back to the interventional radiology suite where both catheters and introducer needles were removed. Expandable hydrogel plugs were delivered at the sub-pleural lung and across the visceral pleura during catheter removal (BioSentry Tract Sealant, Merit Medical, Tarzana, CA, USA). The patient was found to have a small asymptomatic pneumothorax that spontaneously resolved without escalation of care, and was discharged home later the same day.
The patient was subsequently monitored with serial surveillance CT scans every 3 to 6 months as per National Comprehensive Cancer Center guidelines following SBRT. Initial images demonstrated atelectasis in the RLL obscuring tumor control determinations. A single surveillance FDG-PET/CT was obtained at 1.5 years post-brachyablation showing multi-focal regions of moderate FDG uptake with a SUVmax 3.7, compared with SUVmax 7.5 prior to brachyablation. Subsequent CT scans did not demonstrate any evidence of disease progression at 2.8 years post-HDR brachyablation procedure and 7.8 years after initial SBRT (Figure 3C).
Discussion
Primary tumor site progression after SBRT for stage I NSCLC is uncommon. Once confirmed by biopsy, best-practice management strategies include a comprehensive re-staging workup and multi-disciplinary review to identify whether the tumor has metastasized, given the impact that would have on the risk-to-benefit ratio of additional local therapy to a region of the lungs previously treated with SBRT. An ESTRO-EORTC consensus paper recently addressed the scarcity of prospective evidence and understanding of underlying radiobiology to guide re-irradiation management strategies. In that paper which utilized a Delphi consensus approach, it was recommended that re-irradiation cases be classified by whether additional radiation therapy would involve geometrical overlap with previously irradiated volumes (type 1), or no overlap of irradiated volumes with concern for toxicity from cumulative doses (type 2) [15].
For an isolated primary tumor site failure that is technically resectable, surgery is the preferred treatment option since it can remove presumed radio-resistant tumor cells with more comprehensive intralobar, hilar, and mediastinal lymph node staging [16]. However, patients treated with initial SBRT are generally poor surgical candidates, and therefore often present with significant risks of perioperative complications for salvage lung resection. Re-irradiation with salvage EBRT offers an organ-sparing approach, although this too can be challenging to deliver safely due to the risks of exceeding dose limits to normal tissues already exposed to previous SBRT.
Percutaneous image-guided ablation of lung tumors provides a highly conformal option for salvage treatment with intra-tumoral placement of temporary applicators under CT-guidance, to deliver HDR brachyablation, cryotherapy, microwave, or radio-frequency energies [17]. Another salvage therapy option is permanent implantation of low-dose-rate (LDR) brachytherapy seeds with a radioisotope source, such as iodine-125 or palladium-103 [18, 19]. In a case series of 5 patients with 8 pulmonary lesions, including 7 previously treated with EBRT, salvage LDR brachytherapy was associated with the need for one re-implantation and no recurrences at follow-up from the final implant, with an average follow-up of 4.8 years [19]. It is notable that tumors greater than 2 cm in diameter treated with any form of percutaneous salvage therapies may require more than one pleural puncture and several sub-pleural repositioning of each needle, to ensure adequate coverage of the tumor target, introducing higher risks of acute treatment-related complications.
Another option for re-irradiation of lung tumors previously treated with EBRT is salvage SBRT that can be delivered with highly conformal techniques (i.e., VMAT) [20]. A meta-analysis of 20 observational studies analyzing 595 patients treated with salvage SBRT after previous EBRT showed 2-year local control rates of 73% with grade ≥ 3 toxicity of 9.5% [21]. Meanwhile, re-irradiation of centrally located or large tumor targets with SBRT pose higher risks of severe complications with grade 5 toxicity rates reported over 30% [22, 23].
Finally, considering any re-irradiation treatment option, a thorough assessment of cumulative dose affecting both the target and surrounding normal tissue is crucial, including time interval between radiotherapy courses and individual patient-related factors in determining the risk-to-benefit ratio. Retrospective data from a single large academic center suggests composite BED3 (α/β of 3 for normal tissue) as a useful tool to predict for toxicity following thoracic re-irradiation [24]. Established dose guidelines for re-irradiation in the thorax have been outlined by the American Radium Society (ARS) and American College of Radiology (ACR) appropriateness criteria [25]. While these comprehensive dose constraint recommendations (expressed in 2 Gy equivalent dose) primarily apply to re-irradiation with EBRT, they can be extended to and are more likely achievable with HDR or LDR brachytherapy planning techniques [20, 26].
Conclusions
Our institution is currently treating several dozen patients each year with percutaneous HDR brachyablation for lung tumors of various histologies through an ongoing collaboration between the departments of radiation oncology and interventional radiology. The case we present here marks our first experience delivering HDR brachyablation as salvage local therapy following initial SBRT for early-stage NSCLC. Our initial experience demonstrates its safety and effectiveness as a potentially curative management option for early-stage NSCLC developing isolated primary tumor site progression after initial SBRT. Further investigations can help better define its safety and efficacy as a non-surgical salvage therapy option for patients with tumor-site progression after SBRT.