Introduction
Lung cancer is the most common malignancy in Poland. In recent years it was diagnosed in about 16 000 men and about 6000 women a year. Out of all malignant neoplasms, lung cancer is the cause of the highest number of deaths among men and women [1]. Worldwide, lung cancer has been the most frequent malignant tumour and the most common cause of cancer-related deaths in the past few decades. In 2012, a total of 1.8 million new cases were diagnosed, accounting for almost 12.9% of all new cancer diagnoses [2].
About 95% of all lung cancers are of 4 histopathological types: adenocarcinoma (about 40% – the most common type in the non-smoker group), squamous cell carcinoma (about 30%), small cell carcinoma (about 15%) and large cell carcinoma (about 10%). Epidemiological data show that the proportion of glandular cancer is steadily increasing [1].
Early detection and accurate diagnosis of the histopathological type of lung cancer plays a crucial role in the therapeutic process. In the differential diagnosis, two immunohistochemical markers are currently employed – napsin A and thyroid transcription factor-1 (TTF-1).
Napsin A, a novel aspartic proteinase of the pepsin family A, was first described in 1998 by Tatnell et al. [3, 4]. It is mostly expressed in type II pneumocytes, where it is associated with surfactant protein B maturation. Its expression in primary lung adenocarcinoma has been widely described and it is a frequently used marker in lung cancer differentiation [3, 5–7].
Thyroid transcription factor 1 (TTF-1), also known as thyroid-specific enhancer-binding protein, is physiologically expressed in thyroid, brain and lung tissue, where it regulates the expression of surfactant A, B, C and Clara cell proteins. Similarly to napsin A, TTF-1 is also commonly employed in lung cancer differentiation [3, 8, 9].
Both TTF-1 and napsin A are extremely useful tools in distinguishing primary and metastatic lung adenocarcinoma.
Although a considerable amount of literature, including two meta-analyses by Qian et al. and Berghmans et al., has been published on the prognostic role of TTF-1 in non-small cell carcinoma patients [9, 10], there is paucity of research on the prognostic role of napsin A in this group of patients and hence still much uncertainty exists about this relationship.
Aim
In this study, we aimed to assess: (1) the sensitivity of TTF-1, napsin A and combined use of both markers in detecting primary lung adenocarcinoma; and (2) the role of TTF-1 and napsin A both alone and in combination as prognostic markers in primary lung adenocarcinoma patients.
Material and methods
Selection criteria, initial examinations, and follow-up
From January 2012 to December 2012, 318 patients were operated on for lung cancer (mostly lung adenocarcinoma or squamous cell carcinoma). The decision of surgery was made on the basis of diagnostic imaging (CT scan, PET-CT scan), endoscopy (bronchofiberoscopy, autofluorescence bronchoscopy, endobronchial ultrasound-guided biopsy) and other examinations as needed (“blind” transbronchial biopsy, transthoracic needle aspiration biopsy). The cardiopulmonary system was evaluated using spirometry, diffusing capacity of the lung for carbon monoxide (DLCO), capillary blood gas screening and electrocardiography (ECG). In some patients, a 6-minute walk test, stair climb test and additional cardiovascular examinations (exercise test, Holter monitoring, coronary angiography) were also performed. Doubtful cases were finally decided on the basis of the predicted post-operative value of the forced expiratory volume in 1 s (ppoFEV1) and predicted post-operative diffusing capacity of the lung for carbon monoxide (ppoDLCO) calculations.
After the surgery, the patients were consulted by an oncologist and depending on the staging of the tumor, according to the 7th edition of the 2009 TNM classification, histopathological examination, the general condition of the patient and accompanying diseases, they were qualified for adjuvant chemo- and/or radiotherapy [11].
Of the initial cohort of 318 patients who were operated on, 59 were included in the study based on the following eligibility criteria: (1) histologically confirmed diagnosis of primary lung adenocarcinoma or its subtype/variant: lepidic, acinar, papillary, micropapillary, solid predominant with mucin production, mucinous, colloid, fetal or enteric; (2) immunohistochemically stained for TTF-1.
Patients’ follow-up was based on the clinical data collected in the Thoracic Surgery Ward of Poznan University of Medical Sciences (data regarding surgery and control visits) and the Polish Group of Lung Cancer databases. The patients were followed up until 14th August 2016 or death. According to these data the 1-, 3- and 4-year survival rates were determined.
Prior to commencing the study, ethical clearance was sought from Poznan University of Medical Sciences Bioethics Commission.
Immunohistochemical reaction for TTF-1
The immunohistochemical reactions for TTF-1 were performed as part of routine diagnostics between January 2012 and December 2012.
Formalin-fixed, paraffin-embedded tissues were cut into 4 μm sections which were then placed on FLEX IHC Microscope Slides (Dako, Agilent). The immunohistochemical reaction was carried out on the Autostainer Link 48 instrument (Dako, Agilent). Following epitope retrieval through the use of EnVision FLEX Targer Retrieval Solution, High pH (Dako, Agilent) for 20 minutes, the slides were incubated with a monoclonal mouse antibody against TTF-1 (clone SPT24, 1 : 200, Leica, Novocastra) for 30 minutes. EnVision FLEX+ Mouse Linker (Dako, Agilent) was used to enhance the quality of the reaction. The reaction was finally detected using EnVision FLEX Kit, High pH (Dako, Agilent).
The slides were assessed by a specialist in clinical pathology (Katarzyna Iwanik – K.I., MD, Ph.D.). The presence of a brown colored reaction in the nuclei of cancer cells was considered positive. Lack thereof was interpreted as a negative immunohistochemical reaction.
Immunohistochemical reaction for napsin A
Formalin-fixed, paraffin-embedded tissue was cut into 4 μm sections, which were then placed on SuperFrost Plus Adhesion Slides (Thermo Scientific). The slides were pretreated with Cell Conditioning Solution 1 (CC1, Ventana) for 30 minutes and subsequently incubated for 16 minutes with a monoclonal mouse antibody against napsin A (MRQ-20 clone, Ventana) on the BechmarkXT instrument (Roche). The immunohistochemical reaction was finally detected using an ultraView Universal DAB Detection Kit (Ventana).
The slides were evaluated by a specialist in clinical pathology (K.I.). A brown colored reaction in the cytoplasm of cancer cells was considered positive. Lack thereof was interpreted as a negative immunohistochemical reaction.
Statistical analysis
Data management was performed in Open Office Calc 4.1.3. Descriptive statistics were carried out in Statistica v. 12 (StatSoft, Inc. (2014). Statistica (data analysis software system, version 12. www.statsoft.com). Kaplan-Meier estimator was used to assess the overall survival; the curves were then plotted and compared using the log-rank test with statistical significance set at the p = 0.05 level. The survival analysis was performed through the use of MedCalc 17.9.7.
Results
Clinical data
The analyzed groups did not differ significantly in age or sex. The clinical data are presented in Tables I and II
Table I
Clinical characteristics of patients included in the study
Table II
T, N, M categories and stage
The percentage of post-operative complications was 20.3%. The most frequent postoperative complication was atrial fibrillation (10.3%). The possible pre-operative occurrence of this complication was excluded by performing ECG before the surgery. The in-hospital mortality rate was 1.69% and there were no intraoperative deaths. The post-operative period ranged from 4 to 22 days with a mean of 8 days.
Sensitivity of TTF-1 and napsin A in primary adenocarcinoma of the lung
Immunohistochemical expression of TTF-1 was evaluated in all of the 59 cases included in the study. Of those, 44 (74.58%) showed a brown colored reaction in the nucleus of cancer cells and were therefore considered positive, while lack thereof was noted in 15 (25.42%) cases. However, only 48 cases were assessed for napsin A as in 11 cases the appropriate formalin-fixed paraffin-embedded (FFPE) tissues were either lacking or they did not contain enough stainable tissue for evaluation of this particular marker. Of the available cases, 29 (49.15%) were positive for napsin A and 19 (32.20%) were negative. Hence the sensitivities for TTF-1 and napsin A, when used separately, were 74.58% and 49.15% respectively.
With a panel of both TTF-1 and napsin A the sensitivity increased to 79.17% as 38 out of 48 cases were positive for either or both of the immunohistochemical markers. As shown in Table III, 10 (20.83%) cases were negative for both TTF-1 and napsin A, 3 (6.25%) were positive for napsin A but negative for TTF-1, 9 (18.75%) were positive for TTF-1 but negative for napsin A, while 26 (54.17%) showed positive immunohistochemical reaction for both TTF-1 and napsin A (Table IV).
Table III
Number of patients expressing TTF-1, napsin A or both
| Parameter | Napsin A (–-) | Napsin A (+) | Total |
|---|---|---|---|
| TTF-1 (–) | 10 (20.83%) | 3 (6.25%) | 13 (27.08%) |
| TTF-1 (+) | 9 (18.75%) | 26 (54.17%) | 35 (72.92%) |
| Total | 19 (39.58%) | 29 (60.42%) | 48 (100.00%) |
Table IV
Number of patients expressing either TTF-1, napsin A or both along with the sensitivity of a panel of both markers
| Parameter | Number of cases (percentage out of total number of cases) |
|---|---|
| TTF-1 (–) Napsin A (+) | 3 (6.25%) |
| TTF-1 (+) Napsin A (–) | 9 (18.75%) |
| TTF-1 (+) Napsin A (+) | 26 (54.17%) |
| Total | 38 (79.17%) |
Survival analysis
According to the aforementioned methodology, the 1-, 3- and 4- year survival rates were determined. The results for 1-, 3-, 4- year survival data were 87.9%, 70.7 %, 58.6% respectively.
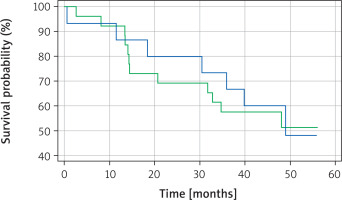
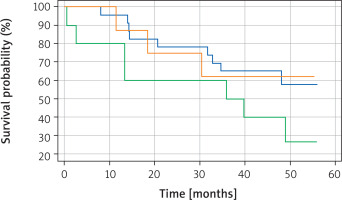
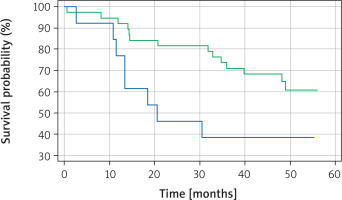
Kaplan-Meier survival analysis was performed for TTF-1, napsin A and for a panel of both TTF-1 and napsin A. There was a statistically significant difference (p = 0.0344) in overall survival between TTF-1 positive and TTF-1 negative patients, with TTF-1 positive patients having a better overall survival (Figure 1). Interestingly, no statistically significant difference in overall survival was noted (p = 0.8814) between napsin A positive and negative patients (Figure 2). Similarly, there was no statistically significant difference in overall survival (p = 0.2196) when cases were compared in the following groups: [1] positive for both TTF-1 and napsin, [2] positive for either TTF-1 or napsin, [3] negative for both TTF-1 and napsin A (Figure 3).
Figure 1
Kaplan-Meier survival curve for TTF-1 positive (green line) and TTF-1 negative (blue line) patients
Discussion
TTF-1 and napsin A are commonly employed antibodies in immunohistochemical differentiation between primary and metastatic lung cancers. In one of the largest studies to date of 1674 cases of lung adenocarcinoma, by Bradley et al. [6], both napsin A and TTF-1 were found to be sensitive markers of primary adenocarcinoma of the lung with sensitivities of 87.0% and 64.0% respectively. In this study, napsin A was described as a more sensitive marker than TTF-1 in detecting primary lung adenocarcinoma. This is in contrast to our study in which TTF-1 was found to be more sensitive (74.58%) than napsin A (49.15%) in detecting primary lung adenocarcinomas. However, according to El-Maqsoud et al. [12] napsin A expression varies with studies and may be as low as 48.5% in non-small cell lung carcinomas. Similarly, following El-Maqsoud et al. [12], TTF-1 expression in different publications is not consistent, with values as low as 39% for lung adenocarcinomas. As shown in our study, combining the use of napsin A and TTF-1 increases the sensitivity of detection of primary adenocarcinoma to 79.17% as it accounts for cases being positive for either or both of the immunohistochemical markers. Therefore, we advocate a panel containing at least TTF-1 and napsin A when diagnosing primary adenocarcinomas of the lung.
To date, two meta-analyses have been performed to analyse the prognostic value of TTF-1 in non-small cell lung cancer. The first one, by Berghmans et al. [10] in 2006, evaluated 10 studies published from 1999 to 2005 with 1101 patients in total. At that time, four out of the ten eligible papers showed that patients with a positive immunohistochemical reaction for TTF-1 in non-small cell lung cancer (NSCLC) have better overall survival but only one showed that a negative immunohistochemical reaction for TTF-1 may be related to poorer survival. The five remaining studies showed no statistically significant difference between TTF-1 positive and TTF-1 negative patients. The combined hazard ratios calculated based on only eight papers, as not all papers had enough data for the meta-analysis, was 0.64, which indicated that TTF-1 positive patients with NSCLC had better overall survival. However, in this meta-analysis only four studies focused exclusively on adenocarcinoma of the lung; out of those, for two the survival analysis did not yield any statistically significant differences, and for the other two better survival was observed in TTF-1 positive patients.
In 2015, another meta-analysis, by Qian et al. [9], was published that covered 17 studies from 1999 to 2012 with a total of 2235 patients. The combined HR for this study was 0.49, and, similarly to the previous meta-analysis, it indicated that TTF-1 positive patients with NSCLC have better overall survival. This study included a greater number of studies with solely adenocarcinoma patients (that is 11 out of 17). The combined HR for the adenocarcinoma subgroup was 0.45, which was consistent with the general conclusion of the study.
In the context of the aforementioned papers, our study also showed a statistically significant difference in survival between TTF-1 positive and TTF-1 negative patients (p = 0.0344). However, recently, a study published by Zhou et al. [13] analysed the difference in survival between 2687 TTF-1 positive and 126 TTF-1 negative patients with stage I adenocarcinoma of the lung. They did not find a statistically significant difference in overall survival between TTF-1 positive and TTF-1 negative patients.
Although the prognostic role of TTF-1 has been studied quite extensively, we have been able to identify only three papers, by Lee et al., Ma et al. and Piljić Burazer et al., that evaluated napsin A as a prognostic factor in patients with a histopathological diagnosis of adenocarcinoma of the lung [14–16]. All three studies showed that napsin A positive patients have statistically significant better overall survival than napsin A negative patients. This is in contrast to our study, which shows that there is no statistically significant difference between the two groups of patients. However, we have been able to assess only 48 cases for napsin A as in the remaining 11 cases the appropriate FFPE tissues were either lacking or they did not contain enough stainable tissue for evaluation of this marker. Hence, it is possible that lack of statistical significance may be attributed to small sample size and further studies are needed to evaluate the relationship between napsin A expression and overall survival.
Similarly to napsin A, there remains a paucity of evidence on the prognostic role of the combined use of both markers – TTF-1 and napsin A. We have been able to identify only one study that analysed the association between combined TTF-1 and napsin A expression and the overall survival. In the study, published in 2015, Ma et al. investigated the relationship between high expression of TTF-1 and napsin A, partial expression of TTF-1 and napsin A, and low expression of TTF-1 and napsin A and overall survival [15]. They found that patients positive for both TTF-1 and napsin A have statistically significant better overall survival. In our study, we also aimed to investigate the association between the combined use of TTF-1 and napsin A and overall survival. In contrast to the study by Ma et al., we divided our patients into three groups: patients positive for both markers, patients positive for either of the markers and patients negative for both markers. We did not find any statistically significant difference (p = 0.2196) in overall survival among these groups of patients.
Conclusions
We have demonstrated that both TTF-1 and napsin A are sensitive markers of primary lung adenocarcinoma with TTF-1 being more sensitive and with the sensitivity increasing when both markers are used in combination. What is more, our study supports the already gathered evidence on the positive prognostic role of TTF-1 in lung adenocarcinoma. However, more studies are needed to elucidate the role of napsin A alone and in combination with TTF-1 as a prognostic marker.