Introduction
Allergic disorders, including asthma, allergic rhinitis and atopic dermatitis have a high global prevalence and severely impact on the quality of life of patients. Furthermore, although the global variety is considerable, these diseases are responsible for high direct and indirect costs [1]. Allergenic sources can vary from biological sources with very complex composition such as pollen, house dust mites (inhalant allergy) or foods (food allergy), to single molecules such as chemicals (occupational allergy) or drugs (drug allergy). It is clear that some proteins are more allergenic than others. Many factors are known to contribute to these differences. Most, but not all, allergens are sensitizing, which is defined as the ability to induce allergen-specific IgE antibodies. Non-sensitizing allergens can only cause allergic symptoms if previous contact with a related (cross-reactive) allergen has caused sensitization. The phenomenon of cross-reactivity between allergenic proteins plays an important role to understand how the immune system recognizes different antigen proteins. Allergen proteins are known to cross-react if their sequence comparison shows a high sequence identity, which also implies that the proteins have a similar 3 dimensional fold [2]. The clinical relevance of immunoglobulin E (IgE) cross-reactivity seems to be influenced by a number of factors, including the immune response against the allergen, exposure and the allergen itself [3]. IgE cross-reactivity often occurs between allergenic molecules in closely related species or well-preserved molecules with similar function present in widely different species, belonging to the same protein family. In addition to major allergens, also minor allergens have been shown to be responsible for cross-recognition of unrelated plant species. Many minor allergens are involved in general vital functions and can therefore be widely found from plants to human. This gives rise to the so called “panallergen” concept [4]. Pollinosis patients often display adverse reactions upon the ingestion of plant-derived foods as a result of IgE cross-reactive structures shared by pollen and food allergen sources. The symptoms of such pollen-food syndromes (PFS) or class 2 food allergies range from local oral allergy syndrome to severe systemic anaphylaxis [5]. Many cross-reactive panallergen components are involved in pollen-food syndromes and associations, such as plant panallergen Profilins, PR-10 proteins (Bet v 1 homologues), and Lipid Transfer Proteins (nsLTPs) [6].
Profilin is a protein of 12–15 kDa in size present in all eukaryotic cells and involved in the organization of cytoskeleton as well as in signal transduction. It is a monomeric actin-binding protein and a key regulator of actin-filament dynamics during processes such as cells movement, cytokinesis, and signalling [7]. In a view of the high sequence homology, cross-reactivity between Profilins is extremely common and involves virtually every plant source [4]. Thus, Profilin can be considered the archetypal pan-allergen. Up to now, 100 Profilins have been described as allergenic, 55 of them in pollen from distant taxonomical species [8]. As for their allergenic potential, they are considered to be incomplete allergens, capable of inducing sensitization by inhalation, but not by ingestion, due to their lability against peptic digestion. Therefore, this syndrome presents with variable frequency in patients with pollinosis and varies by geographic location depending on the primary pollinosis and, probably, allergenic pressure [9].
PR-10 allergens belong to the pathogenesis-related protein groups. They are molecules having a molecular weight of 16–18 kDa, produced in response to biotic and abiotic stresses, and described as allergens in pollen and plant-derived foods. Fifty-three PR-10 have been described up to now, 17 from pollen of the Fagales order, whereas homologous from foods, like apple, carrot, celery, kiwi, are from quite distant taxonomical species [8]. The major birch pollen allergen, Bet v1, represents the prototype of all PR-10-like allergens and is the primary sensitizer in regions with birch pollen exposure. About 50–70% of birch pollen allergic patients, usually after respiratory sensitization, report symptoms after ingestion of fruits and vegetables [10].
Nonspecific lipid transfer proteins (nsLTPs) are a plant defence protein that is highly conserved and present in all plant organs including fruits and is in a greater abundance in the peel than in the pulp of the fruit. nsLTPs are thermally stable and resistant to peptic digestion, as a result of which they behave as full allergens, sensitizing by ingestion and often causing systemic reactions. They are the major allergens of fruits from the Rosaceae family Pru p3, the major allergen of peach, play a precursor role in the sensitization to other nsLTPs. nsLTPs have also been described in pollen such as mugwort (Art v 3), plane tree (Pla a 3), Parietaria judaica (Par j 2) and olive tree (Ole e 7) pollen [11, 12].
A putative primary sensitizer to pollen pan-allergens can be detected only in 1/4 of cases as most patients show IgE specific for > 1 pollen species. In these patients, the prevalence of the primary sensitizer parallels the prevalence of clinical allergy to the different pollen sources in that specific geographic area [13]. Cross-reactivity results in patients having allergic sensitization to many biologically related foods. Accurate diagnosis of the allergens responsible for allergic disease presents therapeutic opportunities for allergen-specific therapies such as allergen avoidance and immunotherapy. In allergy diagnostics, component-resolved diagnostics (CRD) allows the clinician to assess the presence of specific IgE (sIgE) to allergenic proteins (components), instead of crude extracts. Molecular diagnostics has improved our ability to identify clinically relevant cross-reactivity [14].
Aim
The aim of our study was to characterize the Georgian allergic population according to the most frequently recognized plant (pollen and food) panallergen components using sensitization data from multiplex CRD and to investigate their association with particular allergic diseases.
Material and methods
Study population
Data reported herein have been extracted and further analysed from the total of 435 allergic individuals referred for the history of rhinitis, asthma and atopic dermatitis to the Center of Allergy and Immunology (Tbilisi, Georgia) and who have been tested by the multiplex allergy assay. Candidates had to be IgE positive to at least one panallergen component. Selected subjects have been divided in two age groups: children (aged < 18 years) and adults (aged ≥ 18 years).
Allergen Microarray IgE Testing
For the evaluation of polysensitization profiles, the CRD was used. The detection of specific IgE to multiple allergen components was performed by using the 112 component ImmunoCAP ISAC allergen microarray immunoassay (Thermo Fisher Scientific, Uppsala, Sweden). In the present study we focus on the panallergen families and their components: Profilins (Bet v 2, Hev b 8, Mer a 1 and Phl p 12); PR-10 (Bet v 1, Aln g 1, Cor a 1.0101, Cor a 1.0104, Mal d 1, Pru p 1, Gly m 4, Ara h 8, Act d 8, Api g 1); nsLTP (Ara h 9, Cor a 8, Jug r 3, Pru p 3, Tri a 14, Art v 3, Ole e 7, Pla a 3) (Table 1). The measured values ranged from 0.3 to 100 ISU-E, and values ≥ 0.30 ISU-E were considered to be positive results.
Table 1
Demographic and clinical characteristics of the study population
| ISAC positive patients (n = 435) | Cross-reactive panallergen (PR-10, nsLTP, Profilin) reactive patients (n = 164) | ||||
|---|---|---|---|---|---|
| Total | Age group < 18 years old (n = 108) | Age group ≥ 18 years old (n = 56) | P*-values (comparison between age groups) | ||
| Gender: | |||||
| Male | 271 (62.0%) | 109 (66.5%) | 70 (65.0%) | 39 (70.0%) | 0.5 |
| Female | 164 (38.0%) | 55 (33.5%) | 38 (35.0%) | 17 (30.0%) | |
| Age: | |||||
| Min–max | 1–75 | 1–63 | 1–17 | 18–63 | |
| Mean (± SD) | 19.0 (±16.0) | 16.0 (±13.0) | 8.0 (±4.0) | 32 (±11.0) | |
| Clinical phenotypes: | |||||
| Allergic rhinitis | 254 (58.0%) | 109 (66.5%) | 73 (67.0%) | 36 (64.0%) | 0.8 |
| Acute atopic conjunctivitis | 76 (17.5%) | 42 (26.0%) | 26 (24.0%) | 16 (29.0%) | 0.6 |
| Atopic asthma | 65 (15.0%) | 26 (16.0%) | 18 (17.0%) | 8 (14.0%) | 0.8 |
| Atopic dermatitis | 119 (27.0%) | 55 (33.5%) | 41 (38.0%) | 14 (25.0%) | 0.2 |
| Anaphylaxis | 11 (2.5%) | 1 (0.6%) | N/A | 1 (1.8) | 0.3 |
Statistical analysis
All the statistical analyses were performed using IBM SPSS Statistics version 21 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were used with mean, minimum and maximum values to describe continuous variables; absolute number and percentage were reported for categorical variables. Intergroup difference was calculated using the chi square test (χ2). The degree of relationship between the quantitative variables was analysed using the Pearson Correlation test (r). Statistical significance was defined as p < 0.05.
Results
One hundred and sixty-four individuals (38%) of a total of ISAC-positive allergic patients (n = 435) showed an IgE reactivity to at least one molecule belonging to Profilin, PR-10 and nsLTP families. The mean age of selected patients was 16.0 ±13.0. Among them, there were 108 children (mean age: 8.0 ±4.0 years) and 56 adults (mean age: 31.0 ±11.0years). Among children, 65.0% were males and 35.0% were females (male/female ratio 1.8); among adults, 70.0% were males and 30.0% were females (male/female ratio 2.3). In our study group, 109 of 164 (66.5%) were confirmed to have allergic rhinitis, 42 (26.0%) atopic conjunctivitis, 26 (16.0%) asthma and 55 (33.5%) atopic dermatitis. The prevalence of particular clinical entities was similar between two age groups (Table 1).
Generally, PR-10 reactive individuals represented the largest group of patients (92/56.0%), followed by Profilins (70/43.0%) and nsLTPs (53/32.0%). IgE recognition prevalence was broad within PR-10 family members, ranging between 24.0% and 91.0%. IgE sensitization was dominated by Bet v 1 followed by other respiratory and food PR-10 allergens. Sensitization to Gly m 4, Api g 1 and Act d 8, occurred less often. In our study population, IgE recognition frequencies for Profilin homolog components ranged between 47% and 97%. In contrast to Hev b 8 and Betv 2, components such as Mer a 1 and phl p 12 were less frequently detected. Variation of the frequencies of IgE sensitization to different nsLTP homolog molecules were 13–62%. Interestingly, the sensitization to Ole e 7 and Tri a 14 showed lower frequencies in our population. The prevalence ranking of the IgE reactivity to the panallergen molecules was similar for both age groups (Table 2).
Table 2
The frequency of the sensitization to different panallergen components
| Panallergens | IgE reactive patients | P-values* (comparison between age groups) | |||
|---|---|---|---|---|---|
| Allergen components | Allergen source | General population | < 18 years old patients | ≥ 18 years old patients | |
| PR-10 | 92 (56.0%) | 61 (56.5%) | 31 (55.0%) | 0.89 | |
| Bet v 1 | Birch | 84 (91.0%) | 56 (92.0%) | 28 (90.3%) | 0.82 |
| Aln g 1 | Alder | 57 (62.0%) | 37 (61.0%) | 20 (64.5%) | 0.85 |
| Cor a 1.0101 | Hazel pollen | 64 (70.0%) | 42 (69.0%) | 22 (71.0%) | 0.96 |
| Cor a 1.0401 | Hazelnut | 57 (62.0%) | 38 (62.0%) | 19 (61.0%) | 0.87 |
| Mal d 1 | Apple | 63 (68.5%) | 40 (66.0%) | 23 (74.0%) | 0.61 |
| Pru p 1 | Peach | 61 (66.0%) | 39 (64.0%) | 22 (71.0%) | 0.69 |
| Gly m 4 | Soybean | 26 (28.0%) | 19 (31.0%) | 7 (22.5%) | 0.39 |
| Ara h 8 | Peanut | 38 (41.0%) | 25 (41.0%) | 13 (42.0%) | 0.99 |
| Act d 8 | Kiwi | 22 (24.0%) | 14 (23.0%) | 8 (26.0%) | 0.81 |
| Api g 1 | Celery | 25 (27.0%) | 18 (29.5%) | 7 (22.5%) | 0.48 |
| Profilins | 70 (43%) | 46 (43.0%) | 24 (43.0%) | 0.97 | |
| Bet v 2 | Birch | 60 (86.0%) | 39 (84.8%) | 21 (87.5%) | 0.86 |
| Hev b 8 | Latex | 68 (97.0%) | 44 (95.6%) | 24 (100%) | 0.79 |
| Mer a 1 | Annual mercury | 58 (82.9%) | 36 (95.6%) | 22 (91.7%) | 0.45 |
| Phl p 12 | Timothy grass | 33 (47.0%) | 21 (45.6%) | 12 (50.0%) | 0.76 |
| nsLTP | 53 (32.3%) | 38 (35.2%) | 15 (26.8%) | 0.27 | |
| Ara h 9 | Peanut | 28 (53.0%) | 23 (60.5%) | 5 (33.3%) | 0.04 |
| Cor a 8 | Hazelnut | 18 (34.0%) | 14 (36.8%) | 4 (26.7%) | 0.25 |
| Jug r 3 | Walnut | 24 (45.3%) | 19 (50%) | 5 (33.3%) | 0.13 |
| Pru p 3 | Peach | 33 (62.0%) | 26 (68.0%) | 7 (47.0%) | 0.08 |
| Tri a 14 | Wheat | 7 (13.0%) | 6 (16.0%) | 1 (7.0%) | 0.25 |
| Art v 3 | Mugwort | 23 (43.0%) | 15 (39.5%) | 8 (53.3%) | 0.94 |
| Ole e 7 | Olive pollen | 10 (19.0%) | 6 (16.0%) | 4 (27.0%) | 0.68 |
| Pla a 3 | Plane Tree | 21 (38.0%) | 19 (50.0%) | 2 (13.0%) | 0.02 |
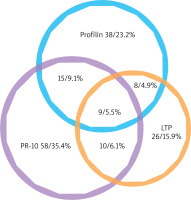
In our study group, 122 of 164 (74.4%) recognized just one out of the three distinct groups of panallergens, with a recognition behaviour: PR-10 allergens 58 (35.4%); Profilins 39 (23.2%); nsLTP 26 (16.9%); only 42 (25.6%) individuals had IgE to more than one panallergen group. Among them, 9 (5.5%) individuals were positive to PR-10, LTP and Profilins concomitantly; 15 individuals (9.1%) to PR-10 and Profilins; 10 (6.1%) individuals to PR-10 and nsLTPs; 8 (4.9%) individuals to nsLTPs and Profilins (Figure 1).
Figure 1
Venn diagram of the plant panallergens reactivity. IgE reactivity distributions of the three panallergen groups: profilins, PR-10, and LTP (n = 164)
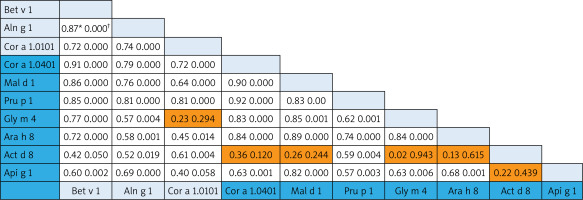
The associations among homolog components belonging to the aero- and food allergens were analysed using the Pearson Correlation test. Since no food components from the Profilin family are present on the ISAC, the Pearson Correlation test was performed only for the PR-10 and nsLTP allergens. In the case of PR-10 molecules, there were strong associations (Pearson Correlation from 0.70 to 0.9) both within and between different food allergens (Cor a 1.0401, Mal d 1, Api g 1,Gly m 4, Arah 8) and aeroallergens (Bet v 1, Aln g 1, Cor a 1.0101). Interestingly, the correlation of food Act d 8 allergens to other food PR-10 family members was less prominent and without statistical significance (except for the correlation of Pru p 1) (Figure 2).
Figure 2
Bivariate analysis of reciprocal relationships of IgE to components of PR-10 family members with p-values (†). Speannan correlation results (*) of the ISU values found for the different PR-10 proteins. Shaded areas in the results field are indicative of statistically insignificant values. The light blue and dark blue color on the allergen lists corresponds to the aero and food components respectively
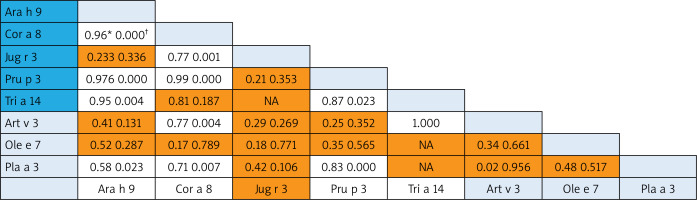
A correlation was revealed for some nsLTP homolog molecules that represented different food and aeroallergens (Pearson Correlation from 0.70 to 1.0): 1) between Art v 3 and Cor a 8 and very strong with rTri a 14; 2) Pla a 3 – correlated with food Ara h 9, Cor a 8 and Pru p 3 (Figure 3).
Figure 3
Bivariate analysis of reciprocal relationships of IgE to components of nsLTP family members with p-values (†). Spearman correlation results (*) of the ISU values found for the different LTP proteins. Shaded areas in the results field are indicative of statistically insignificant values. The light blue and dark blue color on the allergen lists corresponds to the aero and food components respectively
We examined the relationship between sensitization to panallergens and the occurrence of allergic rhinitis, atopic conjunctivitis, atopic dermatitis and asthma. It was shown that sensitization to nsLTPs revealed statistically important associations with allergic rhinitis (p = 0.005) and atopic dermatitis (p = 0.02). PR-10 allergen sensitization was associated with allergic rhinitis (p = 0.04) and asthma (p = 0.04). We did not find any statistically important association between sensitization with Profilins and prevalence of the aforementioned atopic diseases.
Discussion
We studied three groups of molecules belonging to the most prevalent plant panallergen families: PR-10, nsLTPs and Profilins. We did not include polcalcins in our study analysis as only 2.6% of patients (6/234) demonstrated sensitivity to this protein family component.
Our results showed that the sensitization to PR-10 allergens was very common; especially reactivity to the Bet v1 component was prominent. It is a well-known fact that the sensitization greatly depends on the allergen exposure intensity [15]. In Georgia, the birch’s endemic species are scattered in some preserved areas [16] and not in the living area. Based on Georgian pollen count data, birch pollen is detectable in Tbilisi and Kutaisi with a very low total pollen index [17]. Thus, it appears that for our population, alder and hazel-tree pollens cross-reactivity could be a source for PR-10 sensitization.
In opposite to PR-10 allergens, sensitization to the nsLTP homolog molecules showed the lowest prevalence among cross-reactive allergen groups studied in our study population. The prevalence data on peach-allergen-specific sensitization have been investigated in Spanish and Italian studies: lipid-transfer protein (nsLTP) sensitization is predominant in Southern Europe, whereas sensitization to pathogenesis-related 10 (PR-10) is more common in Northern and Central Europe, including areas with Fagales pollen exposure (birch, alder, hazel, hornbeam, oak, beech, and chestnut) [18]. For our population, the Profilin sensitization rate was much higher than this parameter for the nsLTP allergens. Profilins ubiquitous presence and conserved structure in the plant kingdom are at the basis of the acknowledged cross-reactive nature of IgE antibodies against plant Profilins from pollen, plant foods and latex [19].
In our study, the only allergenic components displaying age-group difference were nsLTP components (Ara h 9 and Pla a 3) with the preferential distribution in the paediatric group. Pascal et al. observed that sensitization to Cor a 8 and Ara h 9 occurred almost exclusively in children sensitized to Pru p 3 and Jug r 3. These associations might suggest how sensitization to the different plant-food nsLTPs develops over time, which needs confirmation with prospective studies [20]. Interestingly, Barber et al. also show sensitization towards the peach nsLTP Pru p 3 to be more prevalent within children than in adult populations in areas of high pollen sensitization in Spain [21].
We investigated the prevalence rate for separate allergen molecules (components) within the panallergen families. In case of PR-10 components, the prevalence was prominently higher for aeroallergens (Bet v 1, Cor a 1.0101, Aln g 10) supporting their assumed role as the primary sensitizer [22]. The frequency of sensitization to Bet v 1-related PR-10 food allergens showed the moderate prevalence rates (aver. 61.3% for Mal d 1, Pru p 1, Cor a 1.0401, Ara h 8) and allergens with low prevalence (aver. 26% for Gly m 4, Api g 1, Act d 8). As much as amino acid sequence identities between PR-10 pollen and plant food allergens fall between 38% and 88%, the difference between prevalence should be from their sequence homology and structure similarity [23]. The structural homology between allergenic molecules determines the formation of pollen-food syndromes and associations. Up to 70% of patients with birch pollen allergy can experience a “birch-fruit-vegetable syndrome” [24]. After analysing co-sensitization patterns and the reciprocal relationship between PR-10 components, we found a good association between Bet v 1-related PR-10 aero- and food allergens. Based on our results, Act d 8 (the allergen component from kiwi fruit) showed weak correlation with the most PR-10 food allergens, although it was in good correlation with Bet v 1 and other homolog aeroallergens representing the link to birch-pollen associated kiwi allergy [25].
For the nsLTP family members, we found that food allergen Pru p 3 (allergen component from peach) was the most broadly recognized allergen of the family in our population. In Central Europe, Pru p 3 can be used as the marker allergen for nsLTP sensitization [26]. The consumption intensity of fruits of the Rosaceae family greatly determines the sensitization to the Pru p 3 allergen [27], which is typical for the Georgian population. nsLTPs are a heat and digestion resistant allergen, and these properties make them true food allergens with the capacity of inducing sensitization through the oral route and triggering reactions upon ingestion [28]. It is of note that in contrast to the PR-10 allergen, nsLTP sensitization is present both in patients with and without pollinosis [29]. In this regard, we analysed the co-sensitization pattern within the nsLTP family. The reciprocal correlation revealed weak co-sensitization between Pru p 3 and some aeroallergens (Ole e 7 and Art v 3) and some food allergen (Jug r 3). The first studies by Diaz-Perales et al. found that Art v 3 and Pru p 3 show about 50% identity and a certain degree of cross-reactivity and concluded that this forms the basis of the frequently observed co-sensitization, although mugwort nsLTP shows substantially lower levels of specific IgE binding [30]. In the case of mugwort-peach association, the cross-reactivity appears in a limited group of patients from Southern Europe, in which Art v 3 behaves as the primary sensitizing allergen [31]. Ole e 7 (like Art v 3) displays a sequence identity with plant food nsLTPs of about 50% [32], but Lauer et al. did not observe any correlation between sensitization to peach and olive sources across nsLTP sensitization [33]. In our study, the strong correlation was found between Pru p 3 and Pla a 3. It is of note that this nsLTP component is a major allergen in plane pollen-allergic patients with peach allergy recruited in the Mediterranean area [31]. According to some studies, Pla a 3 showed a variable allergenic potency in comparison with Pru p 3, which led to no general conclusion about the primary sensitizer.
Study analysis revealed that the sensitization prevalence of two Profilin components, Hev b 8 and Mer a 1, was much higher than the sensitization rate of marker Profilin Bet v 2. Interestingly, such results are in line with other studies where it is indicated that these molecules are frequently recognized by patients’ IgE, most likely primarily sensitized by other Profilins. Such findings would suggest carefully considering a molecule as the primary sensitizer merely on the basis of its higher or more frequent IgE reactivity [8].
The relationship between sensitization to panallergens and the occurrence of allergic rhinitis, atopic conjunctivitis, atopic dermatitis and asthma showed some statistically important associations. According of our data, nsLTP was mainly associated with allergic rhinitis and dermatitis in the paediatric population. For the Italian paediatric population it was shown that the manifestation and severity of nsLTP hypersensitivity are extremely variable. Many patients are sensitized although completely asymptomatic; others may show exclusively local reactions, such as contact urticaria or oral allergic syndrome (OAS), whilst others may present more important symptoms such as vomiting, abdominal pains, urticaria-angioedema, asthma and systemic reactions up to anaphylactic shock [18]. The sensitization to the PR-10 allergens was associated with rhinitis and asthma in our paediatric population. According to the cohort study conducted for the Sweden population, IgE response was significantly higher in airborne RP-10 allergens among adolescents with rhinitis and eczema. Among children, sensitization to PR-10 food allergens was found among those with rhinitis [12].
Conclusions
The obtained sensitization pattern to plant panallergens in the Georgian population creates characteristic features with the overlapping serotypes of Central Europe and Mediterranean region. We suppose that it is determined by geographical location, the biodiversity, food and agricultural specificities of Georgia. For the interpretation of the sensitization pattern to cross-reactive allergens, it is utmost important to distinguish a clinically relevant cross-reaction from a cross-reaction without being associated with clinical symptoms. In this regard, studies combining quantitative cross-reactivity measurements at the component level and comprehensive clinical data are needed to establish an evidence-based diagnostic protocol.








