Introduction
Atopic dermatitis (AD) is a chronic inflammatory itching skin disease. Although the incidence of AD is relatively high worldwide, affecting a large number of adults and children, the cause of its onset is not particularly clear [1, 2]. The occurrence of AD may be related to both genetic and environmental factors. Although AD is not considered to be a typical allergic disease, allergens, especially house dust mites (HDM) that are easily exposed to in daily life, may promote the development of AD. Moreover, many patients have elevated IgE, which is also thought to be associated with the onset of AD [3].
Allergen-specific immunotherapy (SIT) is a causal treatment, in addition to improving symptoms, it is thought to slow down the natural progression of the allergy march [4]. Sublingual immunotherapy (SLIT) and subcutaneous immunotherapy (SCIT) are two most commonly used methods in AIT. HDM SCIT has recently been shown to improve eczema symptoms in AD patients in a randomized, double-blind and non-controlled trial [5, 6]. SLIT is considered to be a more convenient and safe treatment alternative to SCIT [7]. However, SLIT has so far been poorly used in the treatment of AD, leaving little clinical evidence of its use in AD patients [8–10].
Aim
The objective of this research was to study the effect of SLIT on HDM sensitized patients with AD in a randomized controlled trial.
Material and methods
Study plan
This is a randomized controlled study with two parallel groups. The patients included in the trial had to be AD patients over the age of 4 years, with a Scoring Atopic Dermatitis (SCORAD) score between 7 and 40, and no persistent bronchial asthma or food allergies. They were randomly divided into SLIT or control groups. Patients in the SLIT group were treated with Dermatophagoides farinae (Der.f.) drops and necessary symptomatic drugs. Patients in the control group could only be treated with symptomatic drugs. The treatment period was 2 years. During the treatment, the SCORAD and a visual analog scale (VAS) score were recorded at regular intervals. A serum level of Der.f.-specific IgE was tested at the baseline and after 24-month treatment. The type and amount of symptomatic drug used was recorded throughout the trial period, and so was the occurrence of adverse events.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national committee and with the 1975 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Patients
Patients of both sexes, aged between 4 and 60 years, were enrolled.
Inclusion criteria were the following: (i) clinical history of chronic AD of over 2 years; (ii) the level of serum Der.f.-specific IgE is class 2 or above (ImmunoCAP; Phadia, Uppsala, Sweden); (iii) during pollen season, no symptom exacerbation was reported; (iv) SCORAD scores greater than 7 but smaller than 40 or less [11].
Exclusion criteria were as follows: (i) bronchial asthma requiring regular treatment with inhaled steroids; (ii) forced expiratory volume in 1 s ≤ 70% of predicted value; (iii) history of actual persistent food allergy; (iv) any previous course of immunotherapy; (v) severe systemic disorders (e.g., cystic fibrosis, diabetes, celiac disease) or malignancies; and (iv) patients who received intercurrent treatment with β-blockers or ACEI during the previous 6 months.
Serum sIgE tests
Serum sIgE tests were performed according to recommendations by using a panel of biologically standardized allergens (ImmunoCAP; Phadia, Uppsala, Sweden) which included house dust mite (Dermatophagoides pteronyssinus, Der.p.; Der.f.), 5-grass mixture (Ambrosia artemisiifolia, Artemisia argyi, Argyranthemum frutescens, Taraxacum mongolicum, Solidaginis Herba), mixed tree, Cladosporium species, and dog and cat allergens.
Treatments
In the control group, treatment with only oral levocetirizine hydrochloride tablets and topical fluticasone propionate are permitted.
Patients in the treatment group were treated with Der.f. drops (CHANLLERGEN, Wolwo Bio-Pharmaceutical Company, Zhejiang, China) for 24 months. According to instructions of the manufacturer of Der.f. drops, the biologically standardized extracts were labelled with the concentration of total Der.f. protein (No. 1, 1 µg/ml; No. 2, 10 µg/ml; No. 3, 100 µg/ml; No. 4, 333 µg/ml; No. 5, 1000 µg/ml). In the first 4 weeks of SLIT, patients were administered increasing doses starting from Der.f. drops No. 1 to No. 4, i.e., D. farinae drops No. 1 were used in the first week, Der.f. drops No. 2 in the second week and Der.f. drops No. 3 in the third week, of those were given respectively as 1, 2, 3, 4, 6, 8, and 10 drops (each drop of 40 µl) day after day in a week. For patients aged 4–14 years, maintenance therapy with three drops of Der.f. drop No. 4 per day from the fourth week to the 24th month. For patients older than 14 years, Der.f. drops No. 4 three drops a day were given in the fourth and the fifth week and then the maintenance therapy with two drops of Der.f. drop No. 5 per day followed from the sixth week to the 24th month. Patients were instructed to keep the Der.f. drops under the tongue for 1–3 min and then swallow. The first dose of Der.f. drops was administered in the physician’s office with specific instructions, and after 30 min observation, patients could leave the hospital. The step-up dosage protocol was standardized as described [12]. Regular oral levocetirizine hydrochloride tablets and topical fluticasone propionate as the control group were added depending on individual diseases.
In the case of cutaneous superinfection, the physician could prescribe a 6-day course of clarithromycin (15 mg/kg/day). No other treatment, including moisturizers, was allowed during the study.
Outcome measures
Patients’ compliance
Patients’ reasons and rate of withdrawal were analysed, and participants failing to complete treatment for 24 months were excluded from the study. The higher the withdrawal rate is, the lower the compliance.
SCORAD score
All the patients were followed up with regular clinic visits during the whole study. The change in SCORAD versus baseline was the primary outcome. The baseline SCORAD was assessed before randomization (run-in period of 1 month) and then after 6, 12, and 24 months of treatment. A Δ SCORAD (difference from baseline) was calculated for analysis.
VAS score
At each visit, patients were asked to mark a line on the VAS. They were asked to quantify the overall AD symptoms on a VAS ranging from 0 (no symptoms at all) to 10 (very severe symptoms). To fill the VAS, they had to answer the question “How was the eczema in the last month?”. As a tool for subjective evaluation, it reflects the quality of life of patients.
Rescue medications score
The patients were required to record drugs’ use in the diary card. The use of medications was scored 1 point for each dose of oral levocetirizine hydrochloride tablets or topical fluticasone propionate and six points for every 6-day course of clarithromycin. The latter was given only in the case of superinfection.
Statistical analysis
Statistical analysis was carried out using SPSS 20.0 software (IBM Corp., Armonk, NY). All tests were 2-tailed, and the level of significance was set at 0.05. The analysis of variance (ANOVA) or Friedman test was used for intragroup comparison (each visit versus baseline). The t-test or Wilcoxon test was used to examine the difference between the treatment group and the control group.
Results
Population characteristics
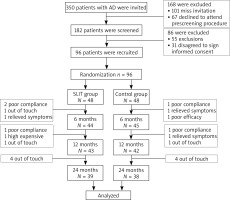
Ninety-six patients, aged between 4 and 60 years (mean age: 26.5 years; 47 males) were enrolled in the study and randomized so that 48 patients were allocated to the SLIT group and the remaining to the control group. All patients had serum sensitization to dust mite. There were no significant differences between the two groups in age, sex ratio, duration, severity, sIgE level at baseline (all p > 0.05; Table 1). 81.3% of the patients (39/48) in the treatment group and 79.2% of the patients (38/48) in the control group had completed the study (p > 0.05). No patients withdrew from the study because of AEs (Figure 1).
Table 1
Demography and clinical characteristics of the enrolled patients at baseline
SCORAD score
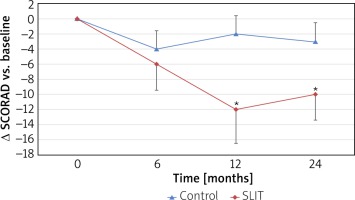
In the SLIT group, the mean SCORAD score decreased from baseline to any post-baseline time point, but in the control group, there is no substantial decrease at 12 and 24 months, as shown in Figure 2. The comparison between groups of changes from baseline revealed statistically significant differences in favour of the SLIT group at month 12 (p < 0.05) and at month 24 (p < 0.05), whereas the difference between groups at month 6 was not statistically significant. The mean (SD) change from baseline to the final visit (month 24) was –10.02 (4.43) in the SLIT group and –2.86 (2.57) in the control group.
VAS score
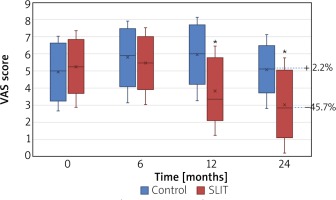
The VAS score of SLIT significantly decreased in the SLIT group compared with the baseline (p < 0.05) and control group (p < 0.05) from month 12, whereas no change versus baseline was observed in the placebo group (all p > 0.05). At the end of the study, an overall increase versus baseline (+ 2.2%) in the control group and a decrease in the active group (– 45.7%; p < 0.01) was observed, respectively (Figure 3).
Rescue medications score
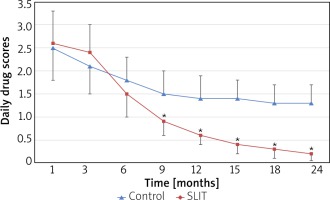
The average daily rescue medications scores were compared at different time points. The SLIT group was significantly lower compared with the control group from month 12 (all p < 0.05). Moreover, at 24-month follow-up, average daily Δdrug scores showed a clear reduction (take the first month as the baseline) in the SLIT group (Δdrug scores, –2.4) compared with the control group (Δdrug scores, –1.2) (p < 0.01) (Figure 4).
Serum Der.f.-specific sIgE level
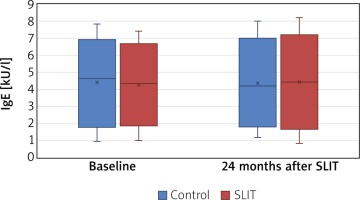
There were no significant differences in serum Der.f.-specific sIgE level between the SLIT and the control group after 24 months (p > 0.05) (Figure 5).
Safety
No patients required hospitalization or withdrew from the study because of AEs. No severe systemic AEs, anaphylaxis, acute attack of asthma, or use of adrenaline were reported. Three patients in the treatment group reported five AEs (3 transient oral itching and 2 gastrointestinal discomfort), and 2 patients in the control group reported two AEs (1 headache and 1 gastrointestinal discomfort). Most of the AEs were grade 1 and were relieved within a week with or without medication.
Discussion
Atopic dermatitis is a common chronic inflammatory skin disease, with a worldwide increase in prevalence, bringing a great impact on the life of patients [13, 14]. The pathogenesis of AD is not yet clear and it is currently believed that AD was related to genetics, environment and lifestyle [15]. Allergens, such as HDM, as an important factor in the environment, promote the onset of AD [16, 17]. From this perspective, SIT may bring some benefits to the treatment of AD.
The role of SCIT in the treatment of AD has been confirmed by several studies. In a recent study, the researchers observed the effect of HDM extract SCIT on patients with HDM-sensitive AD, which showed reduced SCORAD scores and reduced demand for topical corticosteroids [5]. However, the clinical use of SCIT has been limited by site restrictions and safety concerns. SLIT is a safer and more convenient alternative to SCIT, but so far it has limited use in the clinical treatment of AD patients [18]. Recently, only a small number of studies have reported the results of SLIT in patients with AD [8–10, 19, 20]. Our objective was to evaluate the clinical efficacy of SLIT in adult and paediatric patients with AD. The results revealed significant decreases in SCORAD scores and drug intake after SLIT treatment. VAS score also showed an improved trend in the SLIT treatment group, with a significant difference compared with the control group after 12 months of treatment. This is similar to results reported in previous studies.
Currently, there are no recognized serological indicators for the prediction and determination of the efficacy of SLIT in AD [21]. However, some researchers have studied changes in IgE levels before and after SIT treatment, and the results have not been consistent [5, 19, 22]. In our study, levels of allergen-specific IgE in both groups remained unchanged before and after treatment. Further studies may be needed to determine the effects of specific immunotherapy on IgE levels.
SLIT’s safety has been widely acknowledged [23, 24]. The most common adverse reactions are local side effects, including numbness, itching, swelling of the mouth and tongue. In our study, only 3 patients developed transient oral itching or gastrointestinal discomfort, and all were relieved within a week with or without medication. Moreover, no serious systemic reactions or anaphylaxis was observed during the study.
This is the first study of SLIT involving both children and adults with AD in China. Although it shows that SLIT with HDM extracts is effective and tolerable in Chinese children and adult patients with AD, there are several limitations in this study. Firstly, the patients in both groups took medications on-demand, which results in symptom improvement in the control group. In addition, the sample size is small and the observation duration was not long enough. In the future, a large number of confirmatory controlled studies and long-term trials are required in order to verify the effect and safety of SLIT in Chinese patients with AD.
Conclusions
This randomized controlled study has suggested that SLIT to HDM allergen extract could generate significant clinical efficacy for AD patients, as shown by the significant overall reduction in SCORAD scores and the need for rescue medicine. In the future, a large number of confirmatory controlled studies with long-term course are required to reinforce these current results in China.