Dear Editor,
Optimization of tissue perfusion is one of the primary goals of peri-operative care and its adequacy is often assumed based on systemic measures, such as mean arterial pressure (MAP). However, microcirculatory alterations may occur even when global haemodynamic variables are within normal targets and are associated with the development of organ dysfunction. Indeed, perfusion-related complications are a main cause of morbidity and mortality in the surgical population, especially within the first thirty postoperative days [1, 2].
Although several studies have emerged in the last years providing important data that highlight the relationship between intraoperative hypotension and organ injury [3, 4], intraoperative microcirculatory endpoints and the impact of microcirculatory-guided therapeutic interventions have not yet been well defined [5]. In this report, we present a case of intra-operative loss of haemodynamic cohe-rence in a patient with normal systemic haemodynamics during subarachnoid anaesthesia.
A 51-year-old white woman with a body mass index of 33.3 kg m–2, American Society of Anesthesiologists Physical Status Classification II, and stable general condition was scheduled to undergo total knee arthroplasty under subarachnoid anaesthesia. Her medical history was remarkable for well-controlled hypothyroidism and chronic psychosis managed with L-thyroxine (112 μg per day), nebivolol hydrochloride (5 mg per day), trazodone (150 mg per day), olanzapine (2.5 mg per day), agomelatine (25 mg per day), and prazepam (10 mg per day). Physical cardiopulmonary examination before surgery was unremarkable. Preoperative electrocardiogram and laboratory blood tests were also unremarkable except for haemoglobin of 10.6 g dL–1 and creatinine of 0.46 mg dL–1. Preoperative calculated plasma volume status was 0.32.
Upon arrival at the operating room, she was awake with a Glasgow Coma Scale score of 15 and no neurological deficiencies. Standard monitoring was placed. The patient was haemodynamically stable with heart rate 89 beats min–1, arterial blood pressure 126/82 mmHg, respiratory rate 14 min–1, peripheral oxygen saturation 99% on room air, and body temperature 36.4°C. Two large-bore (17G) intravenous cannulae were inserted. She received 5 mL kg–1 of a balanced crystalloid solution to compensate for preoperative fasting and vasodilation associated with anaesthesia followed by an infusion of 2 mL kg–1 h–1.
The patient was placed seated on the operating table with the assistance of one nurse. The puncture area was strictly disinfected and co-vered with a sterile sheet. After anaesthetizing the puncture site with 1.5 mL of 2% lidocaine, the anaesthesiologist performed spinal anaesthesia through the L4–L5 space with a 25G spinal atraumatic needle. When positive reflux of cerebrospinal fluid was observed, a single bolus dose of 2.2 mL (15 mg of 0.75% ropivacaine plus 0.02 mg of fentanyl) was administered. The patient was placed supine and a T7 sensory blockade was confirmed by pinprick test at the 8th minute after intrathecal injection.
Intraoperative systemic haemodynamics remained stable without vasopressor use, and the procedure was evolving smoothly. Approximately 15 minutes after the surgical incision, the patient complained of anxiety and sweating. At that time, a reduction of heart rate (sinus rhythm, 64 beats min–1) was observed with arterial blood pressure being 105/67 mmHg. Arterial blood gas analysis was unremar- kable except for increased lactate (2.7 mmol L–1 [normal range: 0.93–1.65]) and a base deficit of –2.1 mmol L–1 (normal range: –2 to +2).
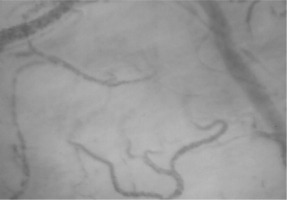
Fifteen minutes later, haemodynamic stability was maintained (sinus rhythm, 67 beats min–1; blood pressure 108/65 mmHg), but the patient complained of anxiety and dry mouth. Based on the available clinical and laboratory data, and after exclusion of other potential causes including surgery-related blood losses, a second sample for arterial blood gas testing was collected, revealing a lactate level of 3.2 mmol L–1 and a base deficit of –3.4 mmol L–1. As the next diagnostic step, sublingual microcirculation was visualized and assessed, revealing plugged vessels next to vessels with flowing cells (type 3 microcirculatory alterations, Figure 1). Taking into consideration the anaesthesia-induced sympathetic nervous system blockade (resulting in a decrease in systemic vascular resistance), the possibility of a subsequent Bezold-Jarisch reflex [6, 7], and the evidence suggesting that impairment of microcirculatory flow should be taken as a serious warning sign for an imminent deterioration [8], the infusion of crystalloids was accelerated and three 50 μg doses of phenylephrine were administered with 5-min intervals (150 μg in total). A second assessment of sublingual microcirculation performed five minutes after the third dose of phenylephrine revealed further deterioration of microcirculatory flow with complete stagnated capillaries (type 1 alterations) in addition to type 3 alterations (Figure 2), suggesting microcirculatory shock due to excessive vasoconstriction. An intravenous dose of 1.5 mg of midazolam was administered to the patient and the radial artery was cannulated and connected to a pressure transducer to directly measure arterial blood pressure. Thereafter, an infusion of glyceryl trinitrate was started at 0.11 μg kg–1 min–1.
FIGURE 2
Aggravation of microcirculatory flow due to excessive vasoconstriction (type 1 and type 3 microcirculatory alterations)
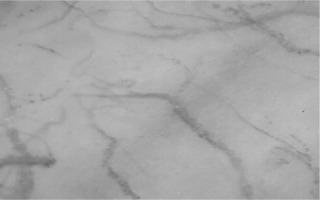
Twenty minutes later, a third assessment of sublingual microcirculation revealed a small improvement in microcirculatory flow (Figure 3). The dose of glyceryl trinitrate was titrated up to 0.46 μg kg–1 min–1, with the blood pressure ranging between 76/54 mmHg and 80/62 mmHg and the patient’s symptoms progressively subsiding without any adverse sequalae during the remaining intraoperative period (Table 1). The surgery was uneventful, and the patient was transferred to the post anaesthesia care unit (PACU). Another assessment at that time (glyceryl trinitrate 0.42 μg kg–1 min–1) revealed improved sublingual microcirculatory flow (Figure 4). The patient remained in the PACU for four hours and she was discharged to the ward after gradual tapering and weaning from glyce-ryl trinitrate infusion. Postoperative calculated plasma volume status at the ward was 3.
FIGURE 3
A small improvement in microcirculatory flow was observed 20 min after the start of glyceryl trinitrate infusion
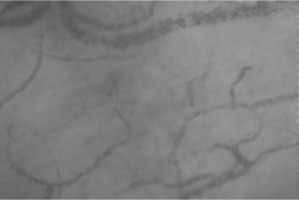
TABLE 1
Perioperative fluctuation of anaesthetic, haemodynamic, and metabolic variables
The postoperative course was uneventful, and the patient was discharged from the hospital after four days. In the follow-up consultations at 6 months, 1 year, and 2 years, she was in perfect condition. No evidence of myocardial, renal, or other injury was observed during hospitalization and follow-up.
Perioperative calculated plasma volume status was obtained by determining actual and ideal plasma volume levels, as previously reported [9]. Mean arterial pressure was initially measured intermittently using a non-invasive oscillometric device every three minutes. Thereafter, MAP was directly measured with a 20-gauge radial arterial catheter connected to the anaesthesia monitor. Before taking measurements, we confirmed that the transducers were correctly levelled and zeroed, while the system’s dynamic response was confirmed with fast-flush tests [10]. Sublingual microcirculation was monitored using SDF+ imaging (Microscan; Microvision Medical BV, Amsterdam, The Netherlands). To optimize video quality, we tried to avoid pressure and movement artefacts, optimized focus and illumination, and cleaned saliva and/or blood from the sublingual mucosa [11, 12].
To our knowledge, this is the first report of microcirculatory shock with type 1 and type 3 alterations during subarachnoid anaesthesia. This case highlights the diagnostic value of intraoperative monitoring of sublingual microcirculation, which may provide information about the mechanisms of tissue hypoperfusion and aid in the determination of an appropriate therapeutic strategy.
Subarachnoid anaesthesia may induce significant systemic haemodynamic impairment due to sympatholytic action and peripheral vasodilation in the blocked area, which decrease systemic vascular resistance and arterial blood pressure. However, its effects on sublingual microcirculation have not been studied extensively. A study investigating the impact of subarachnoid anaesthesia on maternal sublingual microcirculation reported no statistically significant difference in pre- and post-spinal microvascular flow index [13]. Another prospective observational study with patients undergoing elective surgery under subarachnoid anaesthesia reported a significantly decreased microvascular reperfusion rate in the blocked area, although an increase in tissue oxygen saturation was observed due to its sympatholytic effects [14]. In the present case, the impairment of sublingual microcirculatory flow together with the observed metabolic deterioration indicate splanchnic hypo-perfusion [4, 12, 15, 16].
Microcirculatory shock is the failure of microcirculation to support tissue perfusion and oxygenation despite normal systemic haemodynamics [5]. Different types of sublingual microcirculatory alterations have been described. Type 1 alterations include complete stagnated capillaries due to circulatory arrest or excessive use of vasopressors, and/or raised venous pressures. In type 3 alterations, plugged vessels are seen next to vessels with flowing cells (sepsis, haemorrhage, and/or haemodilution). Taking into consideration that the patient was normovolaemic, the most likely cause of microcirculatory impairment was extreme anxiety and the associated catecholamine-induced vasoconstriction. Experimental and clinical research have shown that psychological stress may result in dysfunction of microcirculation, tissue hypoperfusion, and organ damage, especially in women [17–20]. Notably, the microcirculatory impairment was evident in our patient despite the vasodilatory/protective effects of nebivolol and thyroxine [21, 22].
Based on the available perfusion data at the onset of symptoms (normal macrohaemodynamics, capillary refill time, and oxygen saturation), the sympathetic nervous system blockade, the possibility of a Bezold-Jarisch reflex, and the evidence suggesting that impaired microcirculatory flow should be taken as a serious warning sign for imminent deterioration, the anaes-thesia team acted pre-emptively and administered a total dose 150 μg of phenylephrine. The latter further worsened sublingual microcirculatory flow, which was subsequently improved with glyceryl trinitrate infusion. Although the available evidence suggests a non-linear relationship between the macro- and microcirculation, our case indicates a linear relationship until a certain physiological point, beyond which an increase in MAP does not translate into better microcirculatory perfusion.
Intact coherence between the macro- and microcirculation facilitates oxygen delivery to the parenchymal cells. In this context, tissue perfusion is very important because hypoxia does not equate to a specific oxygen concentration. Indeed, many tissues function physiologically at levels equivalent to an atmosphere of 5% oxygen and some at levels as low as 1% oxygen [23, 24]. Furthermore, other phenomena, such as capillary rarefaction or reduced vascular density in individuals with chronic hypertension, may jeopardize tissue oxygenation [10]. The present case confirms that complex mechanisms of haemo-dynamic coherence are required to maintain homeostasis over a very wide range of oxygen concentrations and/or perfusion changes.
Extreme anxiety and psychological stress may impair microcirculatory flow in patients with normal systemic haemodynamics. The present paper highlights the importance of visualization of microcirculation for optimizing perioperative cardiovascular dynamics and improving outcomes.