Introduction
Oesophageal and gastric varices are dilated submucosal veins connecting the portal and systemic circulation, which develop in response to an elevated portal venous pressure. The most common cause of portal hypertension is liver cirrhosis, but it can be caused by abnormalities in the circulation, e.g., thrombosis in the portal vein. The exact prevalence of cirrhosis worldwide is unknown but is estimated to be approximately 1% [1, 2].
In a patient with chronic liver disease, it is important to detect varices at an early stage to prevent a variceal rupture, which can be fatal. Screening for varices should be done at diagnosis of liver cirrhosis, when elastography indicates that advanced liver fibrosis or platelet count is under the lower level of normal in a person with liver disease [3].
Known risk factors for variceal bleeding are large varices or varices with red markings, advanced liver cirrhosis and excessive alcohol use. At diagnosis of liver cirrhosis 50% of the patients have oesophageal varices and approximately 30% of patients will experience bleeding at some point [4].
Over the years 1969-2002, decreased mortality of patients with oesophageal varices was noted in Sweden. For example, when comparing the time periods 1969-1979 with 1990-2002, Stokkeland et al. found that 5-year survival rates increased in men aged < 50 years from 31% to 49% and for women aged < 50 years from 40% to 50% [5].
Furthermore, over the last two decades the mortality of patients with cirrhosis and variceal bleeding has decreased threefold due to improvements in therapy, such as the use of antibiotics prophylaxis, endoscopic therapy, use of vasopressor, restrictive transfusion strategies and transjugular intrahepatic portosystemic shunt (TIPS) procedures [6, 7]. Despite this, variceal bleeding still remains a medical emergency and a major cause of death in patients with cirrhosis. Each bleeding episode is associated with a 15% to 40% mortality rate depending on the degree of liver disease [8]. However, the liver disease per se also contributes to the increased mortality in patients with gastro-oesophageal varices [6, 9].
Due to a more proactive approach to patients with chronic liver disease in the last decades we hypothesized that the survival of patients with varices has further improved.
The aim of this study was to investigate the survival of patients diagnosed with gastro-oesophageal varices at Norrland’s University Hospital (NUS), a regional referral centre in northern Sweden, between the years 2006 and 2019. A secondary aim was to analyse factors associated with survival in these patients.
Material and methods
Patients
All patients with clinical or suspected liver cirrhosis at the medical or infectious departments are referred to screening upper endoscopy for oesophageal varices. In addition, all patients with upper gastrointestinal bleeding are referred for endoscopy. Patients with oesophageal or gastric varices in the region of Västerbotten (approx. 270,000 inhabitants) are all referred to NUS, as it is the only hospital in the region with adequate resources for endoscopic treatment of varices. Oesophageal varices were classified according to Modified Paquet, i.e. grade I varices extending the mucosal level, grade II varices projecting by one third into the lumen and grade III, varices extending more than 50% [10]. Gastric varices were classified according to Sarin et al. (GOV1, GOV2, IGV1 and 2) [11]. Patients who had received a first-time diagnosis of oesophageal or gastric varices (ICD-10 codes I850, I859 and I864) at the endoscopy department at NUS between 2006 and 2019 were identified, including both patients with cirrhotic and those with non-cirrhotic portal hypertension. Exclusion criteria were the following: patients living outside of Västerbotten, non-liver related disease, misdiagnosed varices and patients with confidential medical records. A total of 317 patients were initially enrolled. Among these, 71 patients were excluded from the study. The remaining 246 patients constituted our final study population. A flow chart of the patient selection is shown in Figure 1.
At our hospital we use the Baveno VI protocol in the management of oesophageal varices regarding ligation, glue treatment, antibiotics, terlipressin, and nonselective β-blocker treatment as for when the TIPS procedure is considered.
Study procedures
All data was collected retrospectively from the electronic medical records and traced as far back in time as possible (2006). Records from both the internal medicine and surgical departments were searched. The data were compiled in an Excel database (version 16.48, Microsoft). Data extraction included the variables age at variceal diagnosis, sex, aetiology of portal hypertension and cirrhosis, time of variceal diagnosis, Child-Turcotte-Pugh (CTP) score at variceal diagnosis, time of death, cause of death, compliance with treatment, TIPS procedure, bleeding episodes and liver transplantation. We defined adequate use of non-selective beta blockers based on the proportion of prescriptions recorded in the medical records. Adequate use was defined as > 75%, acceptable > 50% and poor < 50% intake of prescribed drugs.
The aetiology was registered if it was definite or probable and categorized into the following groups: alcohol related liver disease, hepatitis C, alcohol and hepatitis C, cryptogenic, non-alcoholic steatohepatitis (NASH), portal vein thrombosis and autoimmune diseases including autoimmune hepatitis (AIH), primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC). Rare causes, such as haemochromatosis, hepatitis B and α1-antitrypsine deficiency, were grouped together as other causes. Alcohol was considered as the dominant cause if combined with an aetiology other than hepatitis C.
Based on data available in the medical records, patients were regarded as having cirrhosis if liver biopsy results, radiological examinations and/or clinical findings were consistent with that. Compliance with treatment was registered as good, acceptable or bad if mentioned in the medical records. Ascites was diagnosed and categorized according to volume either from a computed tomography scan or from a paracentesis procedure. Hepatic encephalopathy was registered as present if stated so by the treating clinician. All patients were clinically followed until death, emigration or until the end of follow-up, 30 March 2020. Cause of death was obtained from the medical records.
Outcomes
The primary outcome was mortality, defined as time in months from date of variceal diagnosis to date of death. Secondary outcomes comprised the proportion of patients bleeding from their varices, the proportion of patients receiving TIPS and the proportion of patients undergoing liver transplantation. The observed mean age of death was compared to the general life expectancy in the general Swedish population. The average life expectancy of the general population in Sweden was 85 years for females and 81 years for males in 2019 [12].
Statistical analysis
Baseline demographics was presented as medians and ranges for continuous variables and percentages for categorical variables. A two-sided p-value < 0.05 was considered statistically significant. For analysing differences between categorical variables, the Pearson χ2 test was used, whereas for nonparametric analyses between two independent samples of continuous or ordinal variables, the Mann-Whitney U-test was performed. For nonparametric analyses between more than two independent samples of continuous or ordinal variables we used the Kruskal-Wallis H-test. Survival curves were estimated using the Kaplan-Meier method. The log-rank test was used to evaluate differences between overall survival curves. All statistical analyses were performed using IBM SPSS version 26. Missing data were excluded from the statistical analysis.
Results
Cohort characteristics
A total of 246 patients with gastric and oesophageal varices constituted the cohort for the main analysis. Baseline characteristics along with outcomes of the cohort are shown in Table 1. The median age at variceal diagnosis of the whole cohort was 62 (range 17-93) years with no difference between males and females. Alcohol related disease was overrepresented in males (42.3%) while autoimmune diseases were the most common cause of liver disease in females (19.3%) (p = 0.002). Eighty-seven percent (n = 213) of the patients had cirrhosis. Portal vein thrombosis composed the largest group of the patients with varices without an established cirrhosis diagnosis (Table 1).
Table 1
Baseline and characteristics and outcomes of the cohort
[i] Median survival was extracted from the Kaplan-Meier analysis and defined as the time at which 50% of the subjects reached the event (death).
[ii] Autoimmune diseases were grouped and included autoimmune hepatitis, primary biliary cirrhosis and primary sclerosing cholangitis.
[iii] Median survival time could not be estimated for hepatitis C and portal vein thrombosis since less than 50% of the subjects had reached the event at the end of the study.
[iv] Other causes included liver metastasis (n = 4), α1-antitrypsine deficiency (n = 3), unknown cause (n = 3), hepatitis B (n = 3), hepatitis B, C and D (n = 1), Budd-Chiaris syndrome (n = 1), pancreatitis (n = 1), cholangiocarcinoma (n = 1), CVID – common variable immunodeficiency (n = 1), polycystic kidney disease (n = 1), hemochromatosis (n = 1), Mb Banti (n = 1), vascular anomaly (n = 1), Mb Gaucher (n = 1) and side effect of atorvastatin.
At the time of the variceal diagnosis, CTP measurements were evaluable in 97 patients. Of those, 23% were classified into CTP class A, 41% in CTP class B and 36% in CTP class C. We observed a significant difference (p = 0.008) in CTP class depending on aetiology, for which alcohol in combination with hepatitis C, cryptogenic cirrhosis and alcohol related liver disease had the largest proportion of patients in CTP class B or C at variceal diagnosis (100.0%, 92.9% and 86.0% respectively) compared to other aetiologies.
Eighty-two percent of the patients had received treatment with non-selective β-blockers. Compliance (n = 194) was considered good in 80.4% of the patients, acceptable in 12.9% and bad in 6.7%, with no significant sex-related or aetiological differences.
Fifty-nine percent of all patients had received an endoscopic band ligation at some point and 10 patients had been submitted to a TIPS procedure.
Outcomes
At the end of the study, 60.1% of the patients had died at a median age of 69 years (range 26-95). One patient was lost to follow-up. Median survival from the time of variceal diagnosis was 59 months (CI 95%: 45-73 months). Cumulative survival rates at 12, 24, 36, 48, 60 and 120 months were 76.5% (standard error [SE] = 2.9%), 68.6% (SE = 3.2%), 61.6% (SE = 3.4%), 56.6% (SE = 3.5%), 49.7% (SE = 3.7%) and 27.7% (SE = 3.8%) respectively, with no sex-related differences. Survival was significantly lower in CTP class B and C patients compared to CTP class A patients (p = 0.001), as seen in Figure 2. The median survival in CTP class A was 134 months (CI 95%: 0-269 months) compared to 33 months (CI 95%: 19-47 months) in class B and 18 months (CI 95%: 6-30 months) in class C. The 5-year survival was 58.7% (SE = 12.2%), 25.9% (SE = 8.0%) and 23.9% (SE = 7.5%) for CTP class A, B and C, respectively.
Fig. 2
Time in months from variceal diagnosis to death (overall survival), comparing Child-Pugh classes, 99 patients were included in the analysis. Tick marks indicate censored subjects

Survival was also influenced by the aetiological background, as seen in Figure 3 (p = 0.005). Alcohol related liver disease combined with hepatitis C had the lowest 5-year survival rate of 16.7% (SE = 15.2%), followed by cryptogenic cirrhosis with a 5-year survival rate of 45.4% (SE = 10.0%). The NASH and cryptogenic cirrhosis survival curves closely followed the survival curve of the alcoholic liver disease group.
Fig. 3
Time in months from variceal diagnosis to death (overall survival), comparing 4 etiological groups, 128 patients were included in the analysis
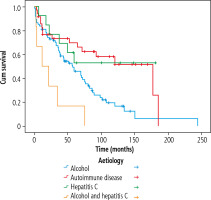
As regards transplant-free survival (Fig. 4), a reduction in survival was seen for the autoimmune disorders and hepatitis C compared to the survival presented in Figure 3 (p = 0.003). The overall 5-year and 10-year survival rates for autoimmune diseases were 69.7% (SE = 8.1%) and 51.5% (SE = 10.2%) compared to the 5-year and 10-year transplant-free survival of 66.5% (SE = 8.3%) and 36.8% (SE = 9.2%). For hepatitis C, the difference between overall survival and transplantfree survival was first observed for the 10-year survival, rates of 52.7% (SE = 14.1%) and 44.0% (SE = 14.3%), respectively.
Fig. 4
Transplant-free survival. Time in months from variceal diagnosis to time of event (liver transplant or death), comparing etiological groups, 128 patients included in the analysis
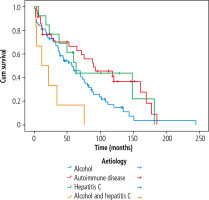
Autoimmune diseases displayed the highest 5-year survival rates in the entire cohort (p = 0.005). The cumulative 10-year survival in the autoimmune disease group was 44.0% (SE = 14.3%) for AIH, 38.1% (SE = 20.4%) for PBC and 75.0% (SE = 21.7%) for PSC. Corresponding numbers for transplant-free survival were 38.6% (SE = 14.0%) for AIH, 31.5% (SE = 14.9%) for PBC and 45.0% (SE = 18.8%) for PSC.
The most common causes of death in our cohort were liver failure (30.3%) and liver cancer (13.5%) with no sex-related differences. 3.1% of the patients died of variceal bleeding. Overall, 31.3% of the patients had one or more episodes of variceal haemorrhage. 19.3% of the patients were diagnosed with varices due to bleeding as a primary symptom. A larger proportion of the men had bled compared to the women, 36.1% and 24.3%, respectively (p = 0.049). Bleeding episodes were more common in some aetiological groups such as portal vein thrombosis (50%) and alcohol related liver disease (41.5%), whereas autoimmune diseases showed the lowest proportion of patients with bleeding episodes (14.3%) (p = 0.039).
We observed a significant difference in overall survival if the patients had bled or not with a median survival of 32 months (CI 95%: 19-45 months) if bleeding had occurred and 79 months (CI 95%: 55-85 months) if bleeding was absent (p = 0.039). The corresponding cumulative 5-year survival rates were 42.2% (SE = 6.2%) and 53.5% (SE = 4.5%), respectively.
The survival was significantly (p = 0.008) decreased if bleeding had appeared as a primary symptom with a median survival of 32 months (CI 95%: 19-45), compared to 70 months (CI 95%: 55-85) if bleeding was not the primary symptom. Corresponding 5-year survival rates were 31.1% (SE = 7.6%) and 54.4% (SE = 4.1%), respectively. Nine (3.6%) of the patients were submitted to a TIPS procedure during the study period.
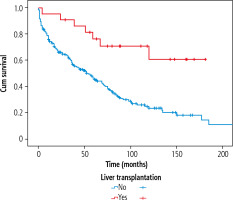
Twenty-eight (11.2%) of the patients had undergone liver transplantation with no difference with respect to sex. Comparing aetiologies, hepatitis C and autoimmune diseases were associated with the highest percentage of patients treated with liver transplantation, 31.3% and 26.2%, respectively (p < 0.001). In the autoimmune disease group, 11.8% of the patients with AIH, 31.3% of the patients with PBC and 44.4% of the patients with PSC had received a liver transplant. According to CTP at variceal diagnosis, 18.2% in CTP A, 19.0% in CTP B and 5.6% in CTP C had been liver transplanted. Liver transplantation significantly improved survival as seen in Figure 5 (p = 0.001). At the end of the study, 31.1% of the liver transplanted patients had died, whereas 67.9% were still alive.
Discussion
This study was based on all patients diagnosed with gastro-oesophageal varices at a single referral centre in northern Sweden, with all major aetiologies in Sweden represented. We found that the mortality of patients with gastro-oesophageal varices significantly exceeds that of the general population in Sweden, despite advances in the management of chronic liver disease and variceal haemorrhage over the years. The mean age of death in our cohort was 70.6 years in females and 68.1 years in males compared to 84.7 years in females and 81.3 years in males in the general population in Sweden [12]. Variceal haemorrhage is a major complication and cause of death in patients with cirrhosis and portal hypertension [8]. In our cohort, however, cause of death was predominantly other liver-related complications rather than variceal bleeding. Only 3.1% of the patients died of variceal bleeding, which is in concordance with a previous study on a similar Swedish cohort [5]. Decreased mortality of variceal bleeding has been observed over the last decades [7]. However, we observed poorer survival in patients with one or more episodes of variceal haemorrhage compared to patients without it, confirming that variceal haemorrhage is still a significant complication associated with high mortality, but rather from complications other than the variceal haemorrhage itself. Variceal haemorrhage is known to have the potential of inducing an acute-on-chronic liver failure, which may be a contributing factor to these findings.
Our results demonstrate a significantly lower survival in CTP class B and C compared to class A, with 5-year survival rates of 58.7%, 25.9% and 23.9% in CTP class A, B and C respectively. This result was expected since a higher CTP score implies a higher degree of liver disease as well as CTP score being a well-known predictor for mortality in cirrhotic patients.
Since liver transplantation significantly improved survival of patients with gastro-oesophageal varices, with 5-year survival rates of 51% and 77.3% in the non-transplant and the transplant group, respectively, our results indicate that liver transplantation should be considered earlier in patients in CTP class B and C with gastric or oesophageal varices.
Our analyses also show that cirrhosis aetiology influences overall survival. Alcoholic liver disease with hepatitis C displayed the worst survival in our cohort, followed by cryptogenic cirrhosis and alcoholic liver disease. All patients with alcoholic liver disease combined with hepatitis C were dead 75 months after their variceal diagnosis. Notably, however, this group was quite small with only six patients included in the analysis. The autoimmune disease group displayed the best survival. These observations are in line with a previous study on a Swedish cohort comparing overall survival in cirrhotic patients by aetiology, in which the lowest 10 year survival rates were found for cryptogenic cirrhosis and alcohol-related cirrhosis, whereas the highest survival rates were found for AIH [13]. That study, however, was not specifically conducted on patients with gastro-oesophageal varices but rather on cirrhotic patients in general. Another Swedish study from 2005 focusing on patients with oesophageal varices reported no difference in mortality comparing different aetiologies [14], which might indicate that changes in therapeutic strategies have impacted the outcome for some of these patients.
The survival curves of patients with NASH and cryptogenic cirrhosis both followed the curve for alcoholic cirrhosis. Cryptogenic cirrhosis has historically been considered a NASH-spectrum disease [15]. However, it might also be considered an alcoholrelated disease. If so, alcohol would be the common denominator for most of the aetiologies with poor survival. There are several reasons for this. First of all, it could be due to a higher CTP class at diagnosis since alcoholic liver disease with concomitant hepatitis C, alcoholic disease alone and cryptogenic cirrhosis showed the largest proportion of patients in CTP B or C in our study. This is in line with a previous study in which alcoholic cirrhosis was reported to have a high prevalence of complications at diagnosis of cirrhosis, such as ascites and encephalopathy, which are both included in the CTP scoring [16].
Another possible explanation is continuous alcohol consumption. In the last decades the behaviour of alcohol use in northern Sweden has gradually transformed from “binge drinking on weekends” to a drinking habit resembling “a Mediterranean drinking pattern” with more regular moderate alcohol consumption [17].
It is known that treatment of the underlying liver disease plays an important role in the management of cirrhosis and varices. In comparison to alcohol-related diseases, some non-alcoholic liver diseases offer better treatment options such as immune modulators for autoimmune diseases or anti-viral treatment for hepatitis. Other possible explanations are treatment compliance and comorbidity. The compliance was examined in this study; however, due to the low quality of data in a large number of patients we could not draw any conclusions from this. We did not gather any data regarding comorbidities in this study. However, a previous study reported a strong association between mortality and burden of comorbidity in patients with cirrhosis [18].
The overall survival was highest in the autoimmune disease group; however, they had a lower transplant-free survival. We observed no significant difference in survival comparing the autoimmune diseases, probably due to the small sample of patients (n = 26) in the analysis. The high overall survival in the autoimmune group could be a reflection of a less progressive disease, due to successful immunosuppressive treatment for AIH, better compliance or less comorbidity, as well the option of liver transplantation. In addition, this group had the lowest incidence of bleeding episodes. Our study shows a 10-year survival of 44.0% in the AIH cohort, which is low in comparison to a study on a Swedish AIH cohort presenting a 10-year survival of 88.2% [19]. Since our study did not include patients without gastro-oesophageal varices, the cohorts are not comparable. However, our data suggest a worsened outcome for patients with AIH in combination with varices, which is probably due to the presence of portal hypertension and cirrhosis and therefore a more advanced liver disease. In the literature it has been demonstrated that cirrhotic patients with presence of gastro-oesophageal varices have a higher mortality and greater risk of decompensation than those without varices [6]. Furthermore, cirrhosis has been shown to predict all-cause mortality [20].
Patients with portal vein thrombosis were not included in the survival comparisons due to the different aetiology of portal hypertension. Interestingly, however, this group had the highest incidence of bleeding episodes, occurring in half of the patients. A previous Swedish study showed a similar pattern but with a significantly longer transplant-free survival for these patients compared to patients with cirrhotic portal hypertension [15].
Our study is retrospective and has the disadvantage that not all laboratory values, signs, symptoms and time of diagnosis could be found in the medical records. Another limitation is the lack of data regarding comorbidities, risk factors, lifestyle factors or other confounders that may have contributed to the mortality. There is a possibility that the physicians who filled out the death certificates, and our own interpretation of the death causes, might be biased because of the knowledge that the patients had liver cirrhosis. Therefore, liver failure as cause of death may have been overestimated in comparison to other causes of death. Unfortunately, during the time of the study, it was not mandatory to document measurements of the varices. Therefore, we did not note the size of oesophageal varices and photographic documentation was nonmandatory.
Our cohort, however, comprises all patients diagnosed with varices at Norrland’s University Hospital. All major aetiologies in Sweden are represented, though a low number of patients in certain subgroups (e.g., PSC and alcohol combined with hepatitis C) limit the accuracy of some parameter estimates such as survival. Another strength is the almost 100% follow-up with only one patient lost to follow-up.
Conclusions
Despite the latest therapeutic advances, the survival of patients with gastro-oesophageal varices remains significantly reduced compared to the general Swedish population. Expectedly, factors associated with poor overall survival are CTP class B and C, alcohol-related and cryptogenic cirrhosis, whereas liver transplantation is associated with a significantly increased survival rate. Our findings suggest that liver transplantation should still be considered earlier in cirrhotic patients with varices and CTP class B and C, and that earlier and more aggressive efforts should be made to achieve alcohol abstinence.