Introduction
In 2020, lung cancer was the second most diagnosed cancer worldwide and the leading cause of death [1]. The 5-year relative survival increased in recent decades, but it remains below 26% [2, 3]. The risk of the disease increases with age and history of smoking [4]. Other risk factors include occupational exposure to asbestos, certain metals, silica, and ionising radiation. In addition, certain chronic respiratory diseases (chronic obstructive pulmonary disease, pulmonary fibrosis, pneumoconiosis, tuberculosis) increase the risk of lung cancer [5, 6].
Wnt is one of the pathways of intracellular signal transduction and is involved in such processes as cell differentiation and proliferation, apoptosis, migration, and invasion. It is regulated by binding of the Wnt ligands to the FZD and LRP-5-6 receptors [7]. Dickkopf-1 (DKK-1) and -2 (DKK-2) belong to the DKK family of proteins, which inhibit the Wnt/β-catenin pathway by binding to LPR5/6 receptors [7, 8]. Changes in activity of the Wnt pathway and associated disturbances in the expression of genes regulated by this signalling pathway lead to multiple pathologies, including cancer [9]. Dickkopf-1 and DKK-2 appear to play dual roles as both proto-oncogenes and tumour suppressors [10]. The role of DKK-1 could be associated with the type of tumour. The role of DKK-1 as a tumour suppressor was observed in tumours arising from endoderm and ectoderm, and that of a driver in tumours arising from mesoderm [11]. The role of DKK-2 as a tumour suppressor and antagonist of the Wnt pathway was confirmed in liver cancer [12], while the oncogenic role was proven in prostate cancer [13]. The role of DKK-1 and DKK-2 in non-small cell lung cancer (NSCLC) subtypes remains unclear. Further research is required for a better understanding of the complexity of their molecular mechanisms and the development of new biomarkers useful in the diagnosis and treatment of NSCLC tumourigenesis. The aim of the study was to analyse the DKK-1 and DKK-2 protein concentrations in NSCLC cancer, including the following 3 subtypes: adenocarcinoma (AC), squamous cell carcinoma (SCC), and large cell carcinoma. The level of DKK-1 and DKK-2 was also analysed in connection with the clinical and demographic variables.
Material and methods
Study group and samples
The characteristics of the study group were described in the previous study [14]. The study group was composed of 65 patients with primary NSCLC. The tumour and non-tumour (NT) samples were collected during surgical resection in the Department of Thoracic Surgery, Faculty of Medical Sciences in Zabrze, Medical University of Silesia in Katowice. The main inclusion criteria were patients over 18 years of age with primary NSCLC, who gave informed consent to participate in the study. Patients who gave no consent or had malignancies other than NSCLC were excluded. The non-small cell lung cancer diagnosis was based on CT-guided lung aspiration biopsy. Positron emission tomography/image-based computed tomography (PET/CT) and CT were used to exclude distant metastasis. The endobronchial/endoscopic ultrasound (EBUS/EUS) was used in individuals showing enlarged mediastinal lymph nodes. R0 resection was performed in all cases. All samples were classified based on the eighth edition of tumour-node-metastasis (TNM) classification of malignant tumours [15]. The tumour samples were verified histopathologically as NSCLC, while the NT samples were confirmed by a pathologist to be free of cancer cells. Non-tumour samples were obtained at a distance of more than 3 cm from the tumour. This study was approved by the Ethics Committee of the Medical University of Silesia in Katowice (PCN/0022/KB1/72/I/20/21). All specimens were frozen at –75°C until further processing.
Homogenisation
Homogenisation was performed according to the methodology described in the previous studies [14, 16]. The samples of the tumour and NT were weighed and homogenised using a PRO200 homogeniser (PRO Scientific Inc., Oxford, CT, USA) at 10,000 rpm in 9 volumes of PBS (EURx, Gdansk, Poland). After homogenisation, sonification with an ultrasonic cell disrupter was performed (UP100H, Hielscher Ultrasonics GmbH, Teltow, BB, Germany).
Total protein measurement and concentrations of Dickkopf-1 and Dickkopf-2
The measurement of total protein was performed according to the methodology described in previous studies [14, 16]. An AccuOrange™ Protein Quantitation Kit (Biotium, Fremont, CA, USA) was used to determine the total protein concentration according to the manufacturers’ instruction. The detection range was 0.1–15 µg/ml. A Synergy H1 microplate reader (BioTek, Winooski, VT, USA) was used to assess the fluorescence at excitation and emission wavelengths of 480 and 598 nm, respectively. The enzyme-linked immunosorbent assay (ELISA) was used to assess the levels of the proteins following the manufacturers’ instructions. The Dickkopf-1 levels were evaluated by a Dickkopf-Related Protein 1 ELISA Kit (Cloud-Clone Corp. Houston, TX, USA; product No. SEA741Hu) with a sensitivity of 0.059 ng/ml. The concentration of DKK-2 was measured with an ELISA Kit for Dickkopf Related Protein 2 (Cloud-Clone Corp. Houston, TX, USA; product No. SEB033Hu) with a sensitivity of 28 pg/ml. For both kits the intra-assay and inter-assay were < 10% and < 12%, respectively. 100 µl of 20´ diluted DKK-1 homogenates and 100 µl undiluted DKK-2 homogenates were used for assay. The absorbance of the samples was determined using a Synergy H1 microplate reader (BioTek, Winooski, VT, USA). The measurement was conducted at the wavelength of 450 nm. The results were obtained with Gen5 2.06 software (BioTek, Winooski, VT, USA). The levels of proteins were normalised to the corresponding total protein level and presented as ng/µg for DKK-1 and pg/µg for DKK-2.
Statistical analyses
The normality of the data was tested using the Shapiro-Wilk test. Results are presented as median with lower and upper quartiles. Student’s t-test and the Mann-Whitney U test were used to verify the significance of differences in means or medians between the groups. To compare the tumour and the NT levels, a paired Student’s t-test was performed. The correlations between the examined variables were determined with Spearman’s rank correlation coefficient. P < 0.05 was assumed as statistically significant. The statistical analysis was performed using STATISTICA 13.1 software (TIBCO Software Inc., Palo Alto, CA, USA).
Results
Study group
The study group was composed of 65 NSCLC patients (mean age 69.09±7.13 years), with 26 (40%) women and 39 (60%) men. Thirty-one (65.96%) cases were current smokers, 16 (34.04%) were former smokers, and 8 (12.31%) were non-smokers. No information on smoking habits was collected from 10 (15.38%) patients. The non-small cell lung cancer patients included 28 (43.08%) with AC, 23 (35.38%) with SCC, and 14 (21.54%) with large cell carcinoma. The clinical characteristics of the study group are presented in Table 1.
Table 1
Clinical characteristics of the study group
Dickkopf-1 and Dickkopf-2 protein levels in non-small cell lung cancer patients
No significant differences were found in the DKK-1 levels in NSCLC tumours compared to NT samples. Analysis of NSCLC subtypes found significantly higher levels of DKK-1 in AC tumours as compared to AC NT samples (p = 0.028). We found that the DKK-2 concentration was significantly lower in tumour samples of NSCLC and all NSCLC subtypes as compared to NT samples. The results are presented in Table 2.
Table 2
Dickkopf-1 and Dickkopf-2 levels in tumour and non-tumour samples in the non-small cell lung cancer group, including adenocarcinoma, squamous cell carcinoma, and large cell carcinoma
The concentration of selected Dickkopf proteins among non-small cell lung cancer subtypes
The comparison of selected DKK protein concentrations among NSCLC subtypes showed that the difference in DKK-1 level in tumour samples was not significant between all subtypes (AC vs. SCC, p = 0.768; AC vs. large cell carcinoma [LCC] p = 0.360; SCC vs. LCC p = 0.383). However, in NT samples, the concentration of DKK-1 was significantly lower in AC compared to SCC and LCC (AC vs. SCC, p = 0.045; AC vs. LCC p = 0.005).
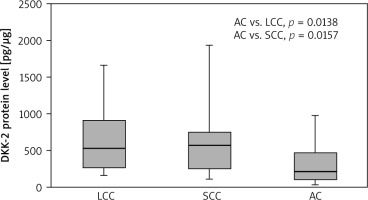
Lower DKK-2 concentrations were found in tumour samples collected from AC patients than in LCC cases (p = 0.045). In NT samples, a lower DKK-2 level was observed in AC cases compared to LCC (p = 0.014) and SCC (p = 0.016). The results are presented in Figure 1 and 2.
Fig. 1
Dickkopf-2 level in tumour samples according to non-small cell lung cancer subtype
Results are presented as box plots with the median in the middle and Q1–Q3 as the box borders where whiskers indicate max and min values; p-values below 0.05 indicated significant differences between groups. AC – adenocarcinoma, DKK-2 – Dickkopf-2, LCC – large cell carcinoma, SCC – squamous cell carcinoma
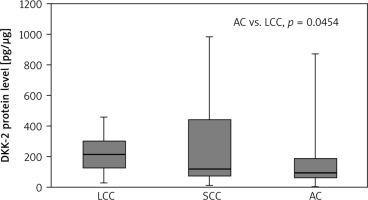
Fig. 2
Dickkopf-2 level in non-tumour samples according to nonsmall cell lung cancer subtype
Results are presented as box plots with the median in the middle and Q1–Q3 as the box borders where whiskers indicate max and min values; p-values below 0.05 indicated significant differences between groups. AC – adenocarcinoma, DKK-2 – Dickkopf-2, LCC – large cell carcinoma, SCC – squamous cell carcinoma
The concentration of selected Dickkopf proteins and sociodemographic and clinicopathological parameters
In the NSCLC subtype group, there were too few patients to analyse the concentration of DKK-1 and DKK-2 according to clinicopathological and socio-demographic parameters. Therefore, we conducted this analysis in the NSCLC group undivided by histological subtypes.
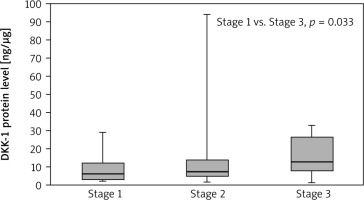
The Dickkopf-1 concentration in the tumour samples was highest in the patients with clinical stage T4 and statistically higher than in patients with T2 (27.14 vs. 6.06; p = 0.048). The results are presented in Figure 3. Moreover, patients with stage III NSCLC showed significantly higher levels of DKK-1 protein in the tumour samples than patients with stage I NSCLC (12.62 vs. 6.04; p = 0.033), which is presented in Figure 4. No other significant differences were found between the concentrations of selected DKK proteins and the socio-demographic or clinical pathological parameters. However, the median concentration of DKK-1 was insignificantly higher in non-smokers compared to smokers (current and former) in tumour samples (non-smoker 9.02 vs. smoker 6.83; current smoker: 5.92; former smokers: 8.65) and in NT samples (non-smoker 8.54 vs. smoker 6.51; current smoker: 6.51; former smokers: 6.17).
Fig. 3
Dickkopf-1 level in tumour samples according to T classification
Results are presented as box plots with the median in the middle and Q1–Q3 as the box borders where whiskers indicate max and min values; p-values below 0.05 indicated significant differences between groups.
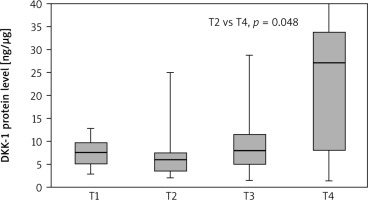
Fig. 4
Dickkopf-1 level in tumour samples according to clinical stage
Results are presented as box plots with the median in the middle and Q1–Q3 as the box borders where whiskers indicate max and min values; p-values below 0.05 indicated significant differences between groups.
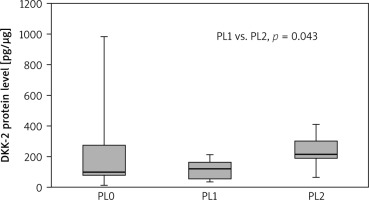
Significantly higher DKK-2 concentrations were found in tumour samples obtained from patients with pleural invasion PL2, compared to PL1 (215.54 vs. 123.13; p = 0.042). The results are presented in Figure 5. We observed higher DKK-2 levels in the tumour samples from NSCLC patients who had never smoked compared to current smokers (185.29 vs. 91.84; p = 0.020). Similarly, in NT samples, the concentration of DKK-2 was significantly higher in patients who had never smoked compared to current smokers (873.99 vs. 252.66; p = 0.036), also when compared to the overall status of a smoker (current + former smoker) (873.99 vs. 315.82; p = 0.042). We observed no other statistically significant differences between the DKK-2 concentration and sociodemographic or clinicopathological parameters.
Fig. 5
Dickkopf-2 level in tumour samples according to pleural invasion
Results are presented as box plots with the median in the middle and Q1–Q3 as the box borders where whiskers indicate max and min values; p-values below 0.05 indicated significant differences between groups.
Our study found a positive correlation between the concentration of DKK-1 and DKK-2 in tumour samples (p < 0.001, rS = 0.548). A similar positive correlation was observed in non-cancer samples (p < 0.001, rS = 0.665). In addition, the level of DKK-2 in NSCLC specimens was positively correlated with DKK-2 concentration in non-cancer samples (p = 0.004, rS = 0.398).
Discussion
The role of DKK-1 and DKK-2 in NSCLC progression remains unclear. Based on our knowledge, this has been the first study to analyse the concentrations of DKK-1 and DKK-2 in tumour and NT samples obtained from patients with NSCLC subtypes.
In the study, the median DKK-1 protein level was insignificantly higher in NSCLC tumours than in NT samples. However, significantly higher levels of DKK-1 protein in tumour samples, compared to NT, were observed in patients with AC. The difference in DKK-1 concentration among NSCLC subtypes was insignificant. On the other hand, we observed that in NT samples, the patients with AC had lower concentrations of DKK-1, compared to LCC and SCC. Some studies found upregulated DKK-1 levels in NSCLC samples, as compared to matched normal tissue by immunohistochemical staging [17–19]. On the other hand, Western blot analysis showed that the DKK-1 protein had a varied level in 10 human NSCLC cell lines [19]. Kim et al. reported that the protein level of DKK-1 was different in NSCLC cell lines: upregulated in the AC cell line (A459) and downregulated in the LCC cell line (H460) [20]. Moreover, a study based on AC cell lines observed that decreased concentration of DKK-1 inhibited cell migration and invasion, which confirmed the oncogenic role of NSCLC cell lines [21]. Based on our results, we could hypothesise that patients with AC would have poorer prognosis than those with other NSCLC subtypes. However, further studies are required to verify DKK-1 as a prognostic factor in AC patients. In our study, the highest concentration of DKK-1 was reported in advanced NSCLC samples with T4 and stage III. In the literature, different studies have shown various results. Kimura et al. [22] found that DKK-1 levels in patients with lung AC and SCC were not associated with T and N clinical parameters but were negatively correlated with relapse-free survival [22]. On the other hand, Li et al. [17] reported that DKK-1-positive status was correlated with lymph node metastasis and a dismal 5-year survival rate. The authors also analysed the NSCLC cell lines and found that DKK-1 promoted invasion and migration of NSCLC cells [17]. Another study based on NSCLC cell lines and animal models revealed positive association between DKK-1 protein levels and vascular mimicry, confirming the role of DKK-1 in tumour growth, inducing the expression of epithelial-mesenchymal transition-related proteins and tumour cell-associated protein stem cells [23]. Also, Pang et al. suggested that DKK-1 played a prominent role in bone metastasis during NSCLC [24]. Interestingly, different DKK-1 levels among NSCLC cell lines were required to modulate the intracellular reactive oxygen species modulator (ROMO1) protein [20]. Kim et al. observed that a decreased level of DKK-1 in A459 induced an increase in the ROMO level, followed by cell growth inhibition and loss of epithelial-mesenchymal transition, while upregulated DKK-1 in H460 inhibited cell survival and downregulated the ROMO level [20]. Our results show that the concentration of DKK-1 was changed in tumour progression, which may suggest an oncogenic role in NSCLC.
We reported decreased concentrations of DKK-2 protein in tumour samples of all subtypes of NSCLC, compared to NT samples. Comparing NSCLC subtypes, we found that the median DKK-2 concentration was lowest in AC: significantly lower than in LCC in tumour samples, and lower than SCC and LCC in normal tissue samples. To our knowledge, this observation has not been reported before. The literature points to the dual role of DKK-2 in cancerogenesis. Based on our knowledge, only a few studies analysed the DKK-2 level in NSCLC. Song et al. found reduced mRNA DKK-2 by Rho GTPase-activating protein 9 (ARHGAP9) in lung AC tumour samples compared to healthy lung tissue. Moreover, the concentration of DKK-2 was reduced in the lung AC cell line that activated the Wnt/β-catenin pathway [25]. Additionally, Zhang et al. reported the role of DKK-2 as an antagonist Wnt signalling pathway [26]. The authors observed that DKK-2 was directly targeted by miR-657, and a decreased level of DKK-1 was associated with TNM stage, differentiation, and lymph node metastasis in NSCLC patients [26]. The tumour suppressor role of DKK-2 in inhibiting cell proliferation and inducing apoptosis was reported in breast cancer [27] and renal cell carcinoma [28]. Furthermore, reduced DKK-2 protein level was noticed in hepatocellular carcinoma [29], while decreased mRNA DKK-2 by promoter methylation was found in breast cancer [27], cervical cancer [30], and colorectal cancer [31]. On the other hand, the negative role of DKK-2 was shown in colorectal cancer, where the upregulated level of this protein in tumour samples was associated with metastasis and angiogenesis [32], while in prostate cancer cell lines it promoted cell proliferation [33]. Additionally, an increased DKK-2 protein level was observed in tumour tissue of the pancreatic AC, which suggested that higher concentration of this protein could be a tool secreted by tumour cells to modulate immune status [34]. Based on our results, we suggested that DKK-2 was a tumour suppressor in NSCLC. Moreover, we hypothesised that patients with AC could have poorer prognosis than patients with LCC or SCC. However, further studies are needed to verify this hypothesis.
Our study reported that smokers had a significantly decreased concentration of DKK-2, while non-smokers showed insignificantly higher median levels of DKK-1. Moreover, we observed that DKK-1 and DKK-2 had higher levels in tumour samples obtained from former smokers than in those from current smokers. The study based on a lung cancer cell line showed that tobacco smoke exposure induces polycomb-mediated repression of DKK-1, and withdrawal of tobacco smoke from culture media effects re-expression of the DKK-1 [35]. It appears that the use of stimulants may impact protein levels and influence the development of cancer.
The main limitation of our study was the small size of samples. Moreover, we have not yet collected enough data to verify the survival rate. However, we plan to analyse the correlation between the concentration and the survi- val rate for patients with NSCLC. The impact of the analysed proteins may be important for the prognosis of patients with specific subtypes of non-small cell lung cancer.
Conclusions
We conclude that the DKK-1 level was increased in advanced NSCLC, and it might play an oncogenic role in lung AC. On the other hand, our results suggested that DKK-2 could play a tumour suppressor role in subtypes of NSCLC, including AC, SCC, and large cell carcinoma. Moreover, the significant differences in DKK-1 and DKK-2 concentrations between tumour and NT samples in patients with AC could suggest a worse prognosis for this NSCLC subtype. However, further studies are needed to verify this hypothesis and determine the prognostic values of DKK-1 and DKK-2 for NSCLC patients.








