Introduction
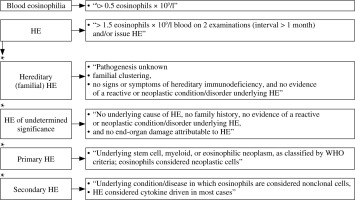
Eosinophils are highly specialized myelopoietic effector cells circulating in peripheral blood that are able to produce and store a number of biologically active molecules. These include major basic protein (PRG2), eosinophil cationic protein (ECP), eosinophil neurotoxin, thromboxane A2 (TXA2), prostaglandins, leukotrienes, and cytokines, such as tumor necrosis factor α (TNF-α) [1–3]. Activated eosinophils release these mediators, thereby influencing homeostasis and tissue integrity [2]. In the case of proliferation and permanent activation, eosinophils can induce inflammatory processes and changes in the microenvironment. This can result in fibrosis or thrombosis with a potential for end-organ damage [2, 3]. In healthy individuals, eosinophils account for about 3% to 5% of leukocytes circulating in peripheral blood. The upper limit of absolute eosinophil count (AEC) in normal peripheral blood is 0.35 × 109/l to 0.5 × 109/l [4, 5]. AEC above this range is considered pathological. The degree of increased eosinophils level is arbitrarily assigned as: mild (higher than upper normal range to 1.5 × 109/l), moderate (1.5 × 109/l – 5 × 109/l), or severe (> 5 × 109/l) [4–7]. Marked and persistent eosinophilia (AEC > 1.5 × 109/l) is referred to as hypereosinophilia (HE) [3, 8]. When irreversible organ damage is involved in HE, the term hypereosinophilic syndrome (HES) is applied [4, 5, 8].
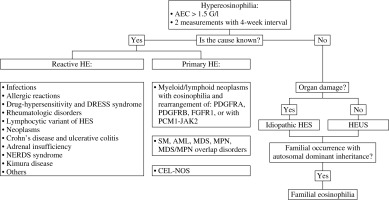
Eosinophilias encompass a broad range of non-hematologic (secondary or reactive) and hematologic (primary, clonal) disorders that may be associated with tissue damage [5, 9]. The pathogenesis of eosinophilia can follow two distinct pathways. Primary eosinophilia is caused by a cell-intrinsic mechanism originating from clonal expansion of eosinophils through acquisition of a somatic mutation, such as that of FIP1L1-PDGFRA. Secondary eosinophilia follows a cell-extrinsic mechanism as a response to exogenous cytokines [6]. In most clinical cases, peripheral blood eosinophilia is secondary (reactive) and is associated with non-hematological disorders such as infections, allergic conditions, connective tissue disorders, vasculitis, malignancy, or endocrinopathies [5, 9, 10]. Because differential diagnosis of eosinophilia is rather complicated, the exclusion of secondary causes should be the first approach. Primary eosinophilia requires not only a thorough medical history and physical examination, but also precise and sophisticated laboratory analyses [11, 12]. Diagnostic tests should include morphologic analysis of the blood and bone marrow samples, cytogenetics and fluorescent in situ-hybridization analyses to detect evidence for an acute or chronic myeloid or lymphoid disorder [5]. The scheme of diagnosis and classification of HE is shown in Figure 1.
The aim of this article is provide an overview of possible causes of eosinophilic disorders based on medical literature. It may support everyday clinical practice, being useful in the differential diagnosis of eosinophilia.
Eosinophils – development and functions
Eosinophils were described and characterized by Paul Ehrlich in 1879 [12]. The human hematopoietic stem cell (hHSC) differentiates into the human common myeloid progenitor (hCMP), on which the interleukin (IL)-5 receptor α chain is not expressed (IL-5Rα−). The hCMP is capable of producing human granulocyte/macrophage progenitors (hGMP), human megakaryocyte/erythrocyte progenitors (hMEP) and human eosinophil lineage-committed progenitors (hEoP). The hEoP cell has a specific phenotype: “IL-5Rα+ (CD125) CD34+ CD38+ IL-3Rα+ CD45RA−” and differs only in the eosinophil line. Probably, part of the hEoP population also derives from the human multipotent progenitor cells (hMPP) and HSC. The process of hCMP differentiation into mature and functional eosinophils is very complex and is based on the interaction of many transcription factors [13, 14]. However, a detailed understanding of eosinophil differentiation and development is the basis for the introduction of targeted therapy in eosinophilic diseases
The best-known eosinopoietic cytokine is IL-5. Its role is important, but it presence is not necessary for the production of eosinophils. Mepolizumab (anti-IL-5 antibody) reduces the amount of mature forms, but not hEoPs [14, 15]. Other cytokines, IL-33, IL-3 and GM-CSF, are involved in eosinophil physiology as well [13, 16]. Eosinophils reside in the hematopoietic and lymphatic organs such as the bone marrow, spleen, lymph nodes, and thymus. In addition, eosinophils physiologically migrate to digestive tract organs, the female reproductive tract, and the mammary gland [17]. They secrete a large number of various cytokines. For instance, IL-4 is involved in immunoglobulin E (IgE) production and the migration of immune cells to the place affected by the inflammatory process in which IL-13 is also involved. Furthermore, IL-13 mediates asthma exacerbations by stimulating mucus production [13, 18]. The capacity of eosinophils for extracellular trap cell death (EETosis) seems to play a significant role in the pathogenesis of eosinophilic disorders. Eosinophils use their own mitochondrial or nuclear DNA in this process [13, 19].
In the course of eosinophilia various organ damage may occur due to cytotoxic activity of eosinophils and secondary fibrosis of affected tissues. In a quarter of patients, thromboembolic complications and organ ischemia are observed [20]. Various forms of skin lesions are the most common manifestation. Cardiovascular diseases, central and peripheral nervous system disorders, pulmonary disruption, gastrointestinal manifestations and others are also possible [20–22].
Up-to-date classification of hypereosinophilia and hypereosinophilic syndromes
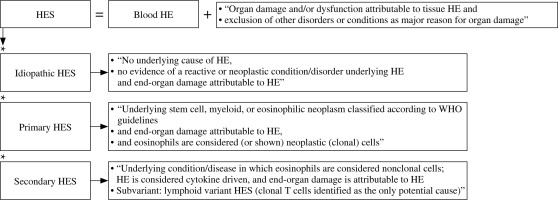
Hypereosinophilia is a heterogeneous group of hematological disorders characterized by marked and persistent eosinophilia in the peripheral blood and tissues [23]. The concept of HES was first proposed by William R. Hardy and Robert E. Anderson in 1968 in an article that reported three cases. The described patients had eosinophilia and organ damage and the diagnosis of eosinophilic leukemia was doubtful [24]. The modern definition of HES is based on the historical criteria outlined by Chusid et al. in 1975: the AEC is > 1.5 × 109/l for more than 6 months, and tissue damage is present. In addition, the underlying cause should be unknown [25].
However, due to new discoveries of pathogenesis, molecular mechanisms and the course of HE a subsequent revision was necessary [20, 24]. The classification developed by the International Cooperative Working Group on Eosinophil Disorders (ICOG-EO) in 2011 is currently in force (Figs. 2 and 3) [8]. The ICOG-EO unified the prior classification criteria and adapted them to the latest molecular biology discoveries. The concept of HE was defined as: AEC > 1.5 × 109/l, confirmed twice with at least a four-week interval. The reason for shortening the time criterion is to avoid complications that may develop over a six-month period. In addition, the concept of tissue HE was determined. This is manifested as bone marrow HE (eosinophils represent > 20% of nucleated cells) or intense tissue infiltration by eosinophils, subjectively evaluated by the pathomorphologist (presence of granule proteins even without massive infiltration of live cells is sufficient). To diagnose HES, it is necessary to show HE in the peripheral blood, regardless of its origin (clonal, reactive or idiopathic) and to identify organ damage or functional disorders that have a direct relationship with HE. At least one clinical manifestation resulting from tissue HE should be proven. Severe eosinophilia can result in thrombosis, neuropathy, cutaneous and mucosal lesions and organ disorders (e.g. liver, kidneys or pancreas involvement). The eosinophil infiltrations or aggregates of granule proteins indicate the association of peripheral blood eosinophilia with the observed pathology. It is necessary to exclude other possible causes of these disorders. Importantly, according to the ICOG-EO, the diagnosis of HES cannot be made when the only clinical manifestation is eosinophilic gastroenteritis, eosinophilic pneumonia, or other examples of single-organ disease listed in the classification (Table 1). In these entities, the role of eosinophils has not been fully elucidated [8, 20]. Initial single-organ manifestation, on the other hand, does not exclude HES. Therefore, definite exclusion of these locations from the HES spectrum seems controversial and unclear. According to Kahn et al., most cases of HE with single-organ involvement should be treated as HES, regardless of the number of organ manifestations and their location [24]. When the disease is life-threatening, the time criterion is unnecessary to recognize HES [8, 20].
Table 1
Organ-restricted conditions in which the effect of eosinophilia remains unclear. Modified table taken from P. Valent et al., 2012 (ICOG-EO 2011)
Clonal eosinophil proliferation leads to primary HES. Secondary causes of HE result in a non-clonal population. A diagnosis of idiopathic HES (HES-I) requires exclusion of all primary and secondary causes of hypereosinophilia and lymphocyte-variant hypereosinophilia [9, 20].
The new concept of hypereosinophilia of undetermined significance (HEUS) has been introduced. Diagnosis of HEUS can be made when no obvious reason is found and no organ damage (necessary to diagnose HES-I) occurs with HE [8, 9]. A hereditary form of eosinophilia is also known. So-called familial eosinophilia (FE) occurs in subsequent generations with autosomal dominant inheritance, probably associated with the 5th chromosome (locus 5q31-q33). In this locus there are IL-3, IL-5 and GM-CSF genes; however, the causative abnormality does not concern them. Organ damage is present only in some cases [20, 26].
Causes of secondary hypereosinophilia
Secondary eosinophilia is defined as all disease entities that cause non-clonal proliferation of eosinophils stimulated by cytokines.
Infections
Numerous parasitic, bacterial and fungal diseases cause an increase in the AEC. Parasitosis should be suspected in the case of immigrants or other people returning from endemic areas. The diagnostic process may be very laborious due to the enormous number of possible disease entities, low sensitivity of stool examination and limited availability of serological and molecular tests. Thus, detailed anamnesis plays an essential role in making a successful clinical diagnosis. However, it should be remembered that a subclinical or atypical course is very common [27, 28]. Enumerating and describing all parasitoses causing eosinophilia goes beyond the scope of this article. Trichinellosis, fascioliasis, schistosomiasis and echinococcosis are the most prevalent disease entities reported in Europe and North America manifested by acute eosinophilia. The etiology of chronic eosinophilia may be associated for instance with strongyloidiasis, clonorchiasis, opisthorchiasis and paragonimiasis [28].
Trichinella spiralis infestations have a global reach. The risk factor is pork, venison or bear consumption [28]. Thermal treatment of food eliminates larvae and prevents infection [29]. The AEC usually exceeds 1 × 109/l. Symptoms, i.e. fever, myalgia and facial swelling, develop after around 1-4 weeks of exposure (from a few days to even 1-2 months) [28, 30, 31]. The mature forms of Fasciola hepatica live in the bile ducts and are manifested by elevated transaminase and bilirubin levels. Infestation occurs through the consumption of feces-contaminated water [28, 32]. Fever, HE and abdominal pain are the main acute phase symptoms. Fasciola is a cosmopolitan parasite; it is noted that 2.6 million humans are infected [28, 32]. Acute schistosomiasis, called Katayama fever, is the result of skin penetration by cercariae (residing in contaminated water) and subsequent migration of schistosomulae. HE (even 7 × 109/l) may be accompanied by flu-like symptoms, cough and diarrhea [28, 33]. Chronic schistosomiasis usually occurs with mild eosinophilia, which is present in half of the cases. Depending on the fluke species it can cause hepatosplenic manifestation, with fibrosis and portal hypertension, the pulmonary form, with angiopathy and secondary right heart failure, as well as genitourinary symptoms. Anyone returning from Africa with fever and eosinophilia should be screened for schistosomiasis [28]. Echinococcosis, the presence of cysts in various organs, is rarely a cause of eosinophilia. It occurs following hydatid cyst rupture, especially spontaneous leakage of contents into the lungs or bile ducts. An atypical clinical picture is a source of frequent misdiagnosis. Rapid iatrogenic perforation of the cyst wall initially causes neutrophilia and the AEC is initially within the normal range. Eosinophilia gradually rises over the next few days. A cyst rupture is a very serious condition that can cause anaphylaxis [28, 34-36]. Strongyloides stercoralis is the most frequent etiologic factor causing chronic eosinophilia. It is usually mild [42]. The disease can be asymptomatic or cause gastrological and cutaneous symptoms (larva currens). Other organ manifestations are also observed due to larval wandering [28]. Ascaris lumbricoides infestation is rarely symptomatic. In chronic ascariasis eosinophilia is infrequent. An acute infection can occur as an eosinophilic pneumonia (Loeffler’s syndrome) caused by larval migration [28, 38]. Other helminths (e.g. Ancylostoma duodenale and Strongyloides stercoralis) can also lead to Loeffler’s syndrome. Nevertheless, there are other causes of eosinophilic pneumonia-neoplasms, drugs and inflammatory diseases. Parasitosis is an important entity in differential diagnosis of asthmatic symptoms with peripheral eosinophilia, notably in endemic areas. In the case of an infectious etiology, steroid therapy impairs the immune system and leads to escalation of symptoms [38]. In conclusion, increase in the AEC requires parasitological diagnosis. Nonetheless, absence of significant eosinophilia does not exclude parasitic disease [28, 38].
The possibility of fungal infection should also be considered. Importantly, different location of lesions is possible, for instance, the digestive tract (e.g. basidiobolomycosis) and respiratory system (e.g. bronchopulmonary aspergillosis or coccidioidomycosis) [28].
Bacterial infections can also occur with HE [39]. The causative relationship seems to be confirmed by the AEC reduction after treatment of Lyme disease. To date, only one case of cerebrospinal fluid eosinophilia has been described [40]. Other reports indicate the possibility of transient synovial fluid eosinophilia in borreliosis-associated arthritis and diffuse fasciitis with eosinophilia [41]. Similarly, the relationship between tuberculosis and peripheral HE is not completely resolved. However, a decrease in the AEC after anti-tubercular treatment was observed [42].
Although eosinophilia is frequently observed in HIV-infected patients, it is usually related to secondary diseases due to immunodeficiency. Insightful diagnostics is important because of the tendency to aggressive infections and cancer development [28, 43].
Allergic diseases
The exclusion of allergic diseases is an extremely important element of differential diagnosis of eosinophilia. Asthma is defined as airway hyperresponsiveness characterized by inflammation and chronic course. Bronchial hyperresponsiveness causes reversible obstruction, with the possibility of becoming persistent [18]. Inflammation occurs with the predominance of neutrophils or eosinophils; the latter better responds to steroid therapy. Atopic asthma is known to have an allergic etiology and specific IgEs are detected in plasma. Furthermore, the response to inhaled steroids is better than in non-atopic cases. Sputum eosinophilia is strongly associated with allergic-type and correlates with the asthma severity [18, 44]. Excluding exacerbations, the AEC is within the normal range or slightly elevated. Moreover, non-allergic asthma with adult onset can be manifested by a HE [18, 45]. Allergic fungal airway disease is another lung disorder accompanied by eosinophilia [18]. Aspergillus fumigatus, Candida albicans and Penicillium species are the main etiological factors. Sensitization coexists with asthma, chronic obstructive pulmonary disease and cystic fibrosis [46]. Allergic eosinophilic esophagitis (AEE), atopic dermatitis (AD) and others can also lead to an increase in the amount of eosinophils. Moreover, AEE and AD are interrelated. Some patients with AEE have AD features [47, 48].
Drug-induced hypereosinophilia
Medication history is crucial in the differential diagnosis of HE. The hypersensitivity reaction is common especially after antibiotics. Medicines can also cause acute eosinophilic pneumonia [27]. A notable but rare condition is DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome. It is manifested by skin lesions and possible life-threatening systemic symptoms [49]. DRESS syndrome was first described in 1930 [49] but the current terminology and first classification criteria of this condition were established in 1996 [50]. Skin lesions are the most characteristic manifestations. However, they are very heterogeneous, from morbilliform maculopapular rash to blisters and erythroderma. The lesions are usually located on the skin above the diaphragm, sometimes also on mucous membranes. Systemic symptoms include fever, lymphadenopathy, hepatic failure, thyroiditis, myositis, myocarditis, pericarditis, uveitis, polyneuropathy, encephalitis and meningitis [49]. Eosinophils and atypical lymphocytes are visible in peripheral blood film. The most common causes of the DRESS syndrome are taking antiepileptic drugs, sulfonamides and antivirals. Other described medicines include allopurinol, proton pump inhibitors, strontium ranelate and minocycline [49, 51]. The pathogenesis of the disease has not been fully elucidated. However, there are four potential causative mechanisms. First, the pharmacogenetic theory is based on human leukocyte antigens (HLA) polymorphism. Certain HLA mutations are potential risk factors for drug-induced cutaneous adverse reactions [49]. The second mechanism is based on the hypersensitivity reaction (type IVb). Drugs as very small particles have the properties of haptens or prohaptens. Eosinophils, basophils and mast cells are activated by IL-4, IL-5 and IL-13 (Th2 – cytokines). Similar to the previous, p-i (pharmacologic interaction with immune receptors) concept which assumes that the drug molecules activate effector cells by forming direct noncovalent bonds with T cell receptor (TCR) or HLA [49, 52]. The last proposed theory is an infectious etiology. DRESS syndrome may be associated with herpesvirus reactivation (i.e. Epstein-Barr virus [EBV], cytomegalovirus [CMV], human herpesvirus [HHV]-6, HHV-7). This relationship is noticeable in 60% of cases. Nonetheless, recent analyses indicate that this causation may also be secondary to immune stimulation by the drug [49, 53]. The first symptoms occur after 2 weeks or even 2 months after the exposure [51]. This may confirm the theory of delayed hypersensitivity, in which T lymphocytes need 4-21 days for activation and expansion [49].
Rheumatologic disorders
Rheumatic diseases particularly associated with eosinophilia include: eosinophilic granulomatosis with polyangiitis (EGPA), immunoglobulin G4-related disease (IgG4RD), diffuse fasciitis with eosinophilia (DFE), eosinophilia-myalgia syndrome (EMS) and eosinophilic myositis (EM). Furthermore, eosinophilia is observed in numerous other conditions, i.e. rheumatoid arthritis, systemic lupus erythematosus, Sjögren syndrome, systemic sclerosis, Behçet’s disease, polymyositis, dermatomyositis, inclusion body myositis, and granulomatosis with polyangiitis [54].
EGPA (formerly Churg-Strauss syndrome) is characterized by allergic manifestations, asthmatic symptoms, vasculitis (inflammation of small and medium vessels), and peripheral and tissue eosinophilia with the formation of granulomas. Vasculitis is manifested by the involvement of the skin, kidneys, heart, peripheral nerves and other organs. The significant increase in the AEC seems to be strongly involved in the pathogenesis of EGPA. Furthermore, an increase in IL-5 level is also observed and the efficacy of mepolizumab seems to confirm this theory [54, 55]. Antineutrophil cytoplasmic antibody (ANCA) is not symptomatic for EGPA due to the presence in other disease entities. Patients with EGPA are mostly ANCA-negative [54, 56]. Differentiation with HES is an important part of diagnostics. Both entities are very similar, especially when there is no ANCA [57]. The prognosis is quite good. The five-year survival rate is 92% [54].
IgG4RD is a broad spectrum of autoimmune disorders characterized by infiltration of IgG4 (+) plasma cells and fibrosis of affected tissues. Tissue or peripheral eosinophilia may also be present. Interestingly, an increased serum level of IgG4 does not occur in all cases and is not required for diagnosis [58].
DFE is manifested by deep induration of subcutaneous tissue and fissures, especially on the limbs. Differentiation with scleroderma is necessary, although the features of both diseases can overlap. Some cases of morphoea profunda may be incorrectly described as DFE. Peau d’orange appearance (orange peel skin) and the groove sign are characteristic symptoms of DFE. The skin induration is preceded by swelling and erythema. The previous nomenclature – eosinophilic fasciitis – is incorrect because tissue infiltration of eosinophils is not required for the diagnosis [54].
Another medical entity with a similar clinical course is EMS. Peripheral eosinophilia and myalgia are the main manifestation of the disease. EMS may also be accompanied by edema, skin lesions, arthralgia, neuropathy, dyspnoea and fever. The most possible trigger factor is L-tryptophan. At the end of the 20th century, there was an epidemic of EMS in the United States. The FDA intervened and withdrew products containing L-tryptophan from sale [54, 59].
Modest data on EM are collected; thus, epidemiology, risk factors and pathogenesis are unknown. EM includes three entities: focal eosinophilic myositis, eosinophilic polymyositis and eosinophilic perimyositis. Eosinophils are probably directly involved in muscle damage and peripheral eosinophilia is one of the proposed diagnostic criteria [54, 60].
Lymphocytic variant of hypereosinophilia
Lymphocyte-variant hypereosinophilia is connected with expansion of the population of T-cells with abnormal immunophenotype (CD3+CD4−CD8− or CD3−CD4+) that produce cytokines, such as IL-5 and in some cases IL-4 and IL-13 [9, 61]. The primary disease manifestations observed in most patients are cutaneous signs and symptoms. The condition is both a clonal process in regards to the abnormal T-cell population and a reactive process because eosinophilia occurs as a reaction to growth factors released by T-cells [9].
Other secondary causes of eosinophilia
Secondary eosinophilia may be the result of hematological or non-hematologic malignancies. Interestingly, the role of eosinophil infiltration in the tumor environment is ambiguous. Tissue eosinophilia is able to promote tumor growth by angiogenesis-stimulating properties. Nonetheless, eosinophils have cytotoxic activity against tumor cells. Moreover, activated eosinophils accompanied by cancer-specific CD8+ T cells support the anti-tumor activity of the immune system. The participation of eosinophils in T-cell chemotaxis, macrophage polarization and even inhibition of neovascularization has been described. Peripheral eosinophilia has been found to correlate with the metastatic process in some studies [62, 63].
Furthermore, inflammatory bowel diseases (e.g. Crohn’s disease or ulcerative colitis) coexisting with eosinophilia have a more aggressive course. This is evident in the frequency of hospitalizations and surgical procedures [64].
A dangerous complication of allogenic hematopoietic stem cell transplantation (allo-HSCT) is graft-versus-host disease (GvHD). One of the features of GvHD is peripheral and tissue eosinophilia. It seems that the presence of eosinophils in duodenal biopsies correlates with the disease severity but peripheral eosinophilia is associated with a better prognosis [65].
Primary (Addison’s disease) or secondary adrenal insufficiency causes eosinophilia due to cortisol deficiency. Glucocorticoids have a pro-apoptotic effect regarding eosinophils, but they promote neutrophilia [66, 67].
The existence of nodules, eosinophilia, rheumatism, dermatitis and edema indicates the occurrence of an extraordinary NERDS syndrome. Swelling of hands or feet and osteoarticular pain result from nodules of tenosynovium. In the peripheral blood elevated values of IgE and eosinophil-derived proteins are noted [68]. Moreover, when eosinophilia and angioedema occur, two entities with different pathophysiology should be considered – the episodic angioedema with eosinophilia syndrome (Gleich’s syndrome) and non-episodic angioedema with eosinophilia [69].
Rare causes of peripheral eosinophilia include Kimura’s disease, which is a chronic, inflammatory illness. The most characteristic manifestation is benign nodules in the subcutaneous tissue and enlargement of the lymph nodes. The head and neck are the most common locations. It may also occur on the torso or limbs. Furthermore, lesions in the kidneys, orbit, outer ear and scrotum have also been described. Sometimes it is accompanied by allergic diseases or kidney disorders. Kimura’s disease is often manifested by significant HE and an increase in IgE titers [70, 71].
Classification of primary hypereosinophilia
After exclusion of secondary causes of eosinophilia, the hematological diagnosis should be performed [9]. In hematological disorders eosinophilia can be caused by both intrinsic and extrinsic mechanisms. In stem cell and myelopoietic neoplasms, eosinophils are derived from the malignant clone, whereas in lymphoid neoplasms and reactive states, eosinophilia is usually triggered by eosinopoietic cytokines [1].
In the case of primary eosinophilia, the World Health Organization classification of myeloid neoplasms and acute leukemia applies. In 2008 the classification of eosinophilic diseases was revised by the World Health Organization. A new major category of molecularly defined primary eosinophilias was created – “Myeloid and lymphoid neoplasms with eosinophilia and abnormalities of platelet derived growth factor receptor alpha (PDGFRA), platelet-derived growth factor receptor beta (PDGFRB), or fibroblast growth factor receptor 1 (FGFR1)” [9, 72]. Lymphocyte-variant hypereosinophilia and HES-I, which is a diagnosis of exclusion, were also established [72, 73]. Other subtypes, such as “chronic eosinophilic leukemia-not otherwise specified” (CEL-NOS), are within the major WHO category of myeloproliferative neoplasms (MPNs) [9, 72]. CEL, NOS is defined by the exclusion of other bone marrow neoplasms associated with eosinophilia, such as acute myelogenous leukemia, myelodysplastic syndrome (MDS), systemic mastocytosis (SM), the classic myeloproliferative neoplasms (MPN), and MDS/MPN overlap disorders. It is also necessary to established the absence of the Philadelphia chromosome or abnormalities of PDGFR, FGFR1 and JAK2 (PCM1-JAK2, ETV6-JAK2 or BCR-JAK2 fusion genes) for diagnosis. CEL, NOS is characterized by an increase in blasts in the bone marrow or blood (but fewer than 20%) or by the evidence for clonality in the eosinophil lineage [9, 72, 73].
The current 2016 revision to the WHO classification maintained the division from 2008 [9, 74]. Another new temporary category has been added: “Myeloid/lymphoid neoplasms with PCM1-JAK2”. The fusion gene is formed on the basis of translocation t(8;9)(p22;p24.1). In the absence of this rearrangement of the JAK2 gene, other variants are possible and meet the diagnostic criteria: t(9; 12)(p24.1;p13.2) (ETV6-JAK2 fusion gene) or t(9;22) (p24.1;q11.2) (BCR-JAK2 fusion gene) [9]. Malignancies with rearrangements of ETV6-JAK2 or BCR-JAK2 genes are very infrequent and therefore not distinguished as individual entities. Most often, they were classified as the B-cell ALL variant. The new JAK2 gene rearrangement subunit is provisional and requires further investigation [74, 75].
Importantly, the WHO classification has some crucial limitations. Current criteria based on the cytogenetic and molecular factors preclude logical division according to the histological type. Myeloid and lymphoid neoplasms may be classified as a common molecular category. Furthermore, detection of one driver mutation does not exclude the co-occurrence of others. The hierarchy of molecular changes is not determined. Additionally, coexistence of more than one malignancy is possible. For instance, the coincidence of a neoplasm with rearrangement of FIP1L1/PDGFRA and SM has been described [8]. Thus, recognizing one disease entity does not exclude others [76]. Nevertheless, the molecular division is very valuable because of its prognostic and predictive properties. The rearrangement of PDGFRA/B has a relatively good prognosis. CEL-NOS and the FGRFR1 or JAK2 abnormalities are associated with poor prognosis. The existence of a specific fusion gene also determines the type of recommended treatment [8, 9, 77].
Moreover, it should be remembered that clonal HE may also be associated with other WHO-defined hematological neoplasms, i.e. SM, acute myeloid leukemia (AML), MDS, chronic myeloid leukemia (CML), other MPN and MDS/MPN overlap disorders [8, 9, 78].
Summary
Because of many underlying causes, the diagnosis of eosinophilia and eosinophil disorders is a great challenge not only for hematologists, but all physicians. Advancement in the diagnostics based on molecular criteria, targeted therapy and a greater understanding of the HE pathogenesis had an important impact on the survival of patients with HES. The analysis of the literature indicates a significant improvement in the prognosis. A literature review from the 1970s indicated that only 12% of patients with HES survived 3 years [9, 25]. In the 1980s, the 5-year survival rate increased to 80% [79]. Nowadays it is over 90%, with a significant improvement in long-term prognosis [9]. The continuous developments for better understanding of eosinophilia may improve diagnosis, treatment, and prognosis in this group of disorders.