Introduction
Today, non-alcoholic fatty liver disease (NAFLD) is becoming an emerging public health problem in liver disease [1]. NAFLD can be defined as the condition of steatosis of the liver that results from the accumulation of fat in the liver that is not caused by alcohol consumption and other secondary causes of liver steatosis [2]. The prevalence rate of NAFLD has increased in the last 20 years to reach 25.54% worldwide, with the largest number coming from the Middle East and South America [3]incidence, progression, and outcomes of NAFLD and nonalcoholic steatohepatitis (NASH. NAFLD is thought to be the main cause of chronic liver disease that progresses to nonalcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma (HCC) [4].
Non-alcoholic fatty liver disease mostly occurs in populations with a high prevalence of metabolic syndrome, in which various metabolic factors such as obesity, type 2 diabetes mellitus, and hyperlipidemia are the main risk factors for developing NAFLD [3, 5, 6]. Consumption of red meat and soft drinks and low consumption of fruits and vegetables may increase the risk of NAFLD [7, 8]. Genetic factors play an important role in increasing the risk of NAFLD development and cause more severe histologic liver damage [9, 10]. Men also have a higher risk of NAFLD than women, although postmenopausal women have the same risk of developing NAFLD as men [11, 12].
Interventions with lifestyle modifications with targeted weight loss are the first line in treating NAFLD, but some obese patients develop musculoskeletal problems resulting in limited physical activity [13, 14]. There is no definitive pharmacological therapy for NAFLD other than therapy to reduce the risk factors associated with NAFLD [15]. Some non-specific agents such as glucose-lowering drugs (metformin, peroxisome proliferator-activated receptors γ [PPAR-γ], GLP-1 receptor agonists and a new type of sodium-glucose co-transporter-2 [SGLT-2] inhibitor drugs such as tofogliflozin), lipidlowering agents, statins, antihypertensive agents, and vitamin E have been studied for their effects on improving NAFLD [13, 16]. The use of silymarin, an extract from Silybum marianum, which has anti-inflammatory and antifibrotic effects against NAFLD progression and can be used when hepatic enzymes increase 1.5-2 times can give positive results based on expert opinion [17]. Recently, new therapeutic approaches by modifying the regulation of interferon regulatory factors (IRFs) through various agents to inhibit NAFLD progression still require further study regarding their effect on NAFLD and its progression [18].
Seaweed is a food that is widely consumed in Asia as a food or snack [19]. Several studies have been conducted regarding the health benefits of seaweed, including lipid and glycemic reduction [20]. Seaweed contains various components such as fiber, polyphenols, and various types of antioxidants that can reduce lipid levels in the body that affect its metabolism in the liver [21-23]. Although there is a relationship between seaweed consumption and lowering the risk of NAFLD, the effect of seaweed consumption on NAFLD has not been widely studied [24]. The aim of this study is to compare the effect of seaweed consumption on improving liver injury in NAFLD patients.
Method
Search strategy
Article searches were conducted using PubMed, Google Scholar, and Cochrane from January 2021 to April 2021 based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline. The keywords used were “non-alcoholic fatty liver disease”, “NAFLD”, “seaweed”, “liver enzymes”, “liver fat” and “diet”.
Outcome measures
The primary outcome is the change of liver enzymes (alanine aminotransferase [ALT], aspartate aminotransferase [AST], alkaline phosphatase [ALP] and γ-glutamyl transferase [GGT]) while the secondary outcome included body weight, waist circumference, body mass index (BMI), lipid profile, insulin level and insulin sensitivity and any related metabolic indicators.
Inclusion and exclusion criteria
The inclusion criteria used were types of study being randomized controlled trials (RCTs), cohort studies, and clinical trials, full text in English with publication within the last 12 years. The exclusion criteria used were a review, systematic review, meta-analysis, double reported, non-full text, publication not in English and published more than 12 years ago.
Data extraction
One investigator (MLA) assessed and abstracted the selected studies independently and one investigator (MDP) checked the data extraction. The data extracted from the article were: study (type of study, year of publication, location), patients (number, mean of age, and sex), intervention (length of study, type of intervention and control, and dosage), and outcome (liver enzymes [ALT, AST, ALP, GGT] and any secondary outcome related to risk factor of NAFLD).
Results
Selection and study characteristics
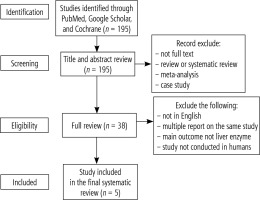
From the literature search, we identified a total of 195 studies. A total of 157 studies were excluded because the article was not in full text, the article was a review or systematic review, meta-analysis, or case study. Most of the studies found were animal studies and did not mention or test liver enzyme levels before and after the intervention. After a thorough review, we report five studies that fit the inclusion criteria (Fig. 1).
All of the studies were conducted on a small sample (N < 100) with a randomized controlled trial (RCT) type of study conducted on NAFLD patients who were not diagnosed with diabetes or undergoing antidiabetic treatment or treatment with other medications. Most of the studies were conducted in Asia [25-28] and one study was from Europe [29]. The study focused on an adult population between 36 and 59 years of age with the majority of the sample being women. The study was conducted in a clinical setting with the intervention carried out in a hospital. The duration of the intervention varied from 12 weeks (3 months) to 8 months. Details of the characteristics of the study are presented in Table 1.
Table 1
Characteristics of the included studies
| Reference | Country | Year of publication | Type of study | Duration | Group | Dropout | Mean age (years) | ||
|---|---|---|---|---|---|---|---|---|---|
| Control | Intervention | Control | Intervention | ||||||
| [29] | Russia | 2010 | RCT | 16 weeks | 36 obese premenopausal women patients with NAFLD | 36 obese premenopausal women patients with NAFLD | 0 patient | 0 patient | 37.4 ±2.8 (control group) and 36.1 ±2.1 (intervention group) |
| [25] | Iran | 2012 | RCT | 3 months | 43 patients | 33 patients | 10 patient | 12 patient | 47.10 ±8.27 (control group) and 51.00 ±7.94 (intervention group) |
| [26] | Iran | 2014 | RCT | 8 months | 26 (15 men) | 26 patients (15 men) | 4 patients | 1 patient | 37.73 ±8.24 (control group) and 37.0 ±7.45 (intervention group) |
| [27] | Taiwan | 2019 | RCT | 12 weeks | 21 patients (11 men and 10 women) | 21 patients (7 men and 14 women) | 1 patient | 1 patient | 59 ±10.5 (control group) and 55 ±12.5 (intervention group) |
| [28] | Taiwan | 2021 | RCT | 6 months | 21 patients (11 men and 10 women) | 21 patients (7 men and 14 women) | 0 patient | 0 patient | 59 ±10.5 (control group) and 55 ±12.5 (intervention group) |
Primary outcome
All studies showed significant improvement of liver injury in the intervention group regardless of the type of intervention and its combination with placebo (Table 2). Three studies [27-29] used extracts from brown seaweed (fucoxanthin and fucoidan) with different study durations (12 weeks vs. 16 weeks) which can reduce liver enzyme levels. Two of the three studies [27, 28] used two different extraction combinations which resulted in different potencies, but the extraction from brown seaweed produced a significant reduction in liver injury. Two other studies [25, 26] used Chlorella vulgaris with different durations (3 months vs. 8 months) and showed significant improvement in liver injury compared with controls using placebo or treatment without Chlorella vulgaris supplementation. Details of secondary outcomes are presented in Table 2.
Table 2
Primary and secondary outcomes of the included studies
Secondary outcome
All studies show that seaweed supplementation can reduce BMI significantly. Two studies [25, 27] showed no significant effect on some parameters from secondary outcomes. Two studies [25, 26] also reported the severity grade of NAFLD in the characteristic of the patient, while two other studies did not report it. The difference in the secondary outcome effect could be influenced by the shorter study duration of the two studies compared to the other studies. In one study [27], the significant change in liver enzyme did not affect CAP, the parameter of liver steatosis. One study [29] reported that there is no adverse effect from all participants while other studies did not report the adverse effect. Details of secondary outcomes are presented at Table 2.
Discussion
This review focuses on assessing liver enzyme levels for the improvement of liver injury in NAFLD patients after treatment with seaweed supplementation. All studies demonstrated the effect of intervention with seaweed supplementation on improving liver injury in NAFLD patients, apart from different types of seaweed and their combination. Differences in the duration of the intervention did not affect the improvement of liver injury in liver enzyme levels, although it may have an effect on secondary outcomes which are associated with risk factors for NAFLD.
Liver enzyme evaluation has been widely used to assess liver function associated with early identification and metabolic risk factors for NAFLD as well as future risk stratification [30]. The use of liver enzymes to assess liver function is also widely used for other liver diseases such as viral hepatitis, Wilson’s disease, drug reactions, and autoimmune hepatitis, making it an issue in the specificity and sensitivity of NAFLD detection [30]. Despite the issues of sensitivity and specificity, liver enzyme assessment is important for the identification of liver injury in metabolic syndrome patients and non-alcoholics [31-33]. If there are indications of NAFLD, imaging studies or liver biopsy need to be done to determine the diagnosis of NAFLD or a possible worsening of NASH to fibrosis [31, 34].
The first line of NAFLD management is weight loss with lifestyle interventions [35]. While it is easy to stress how important weight loss is for NAFLD patients, this goal is difficult for many to achieve [35]. Diet plays an important role in weight loss, which by optimizing micro- and macro-nutrients can help reduce weight [35]. Increasing the number of foods that contain lots of antioxidants such as fruits, vegetables, and edible plants can be a potential therapeutic agent to treat NAFLD [36].
There have been many animal studies in NAFLD models using seaweed for liver injury improvement. A study demonstrated the effect of fucoidan and fucoxanthin in the NAFLD rat model to have the effect of reducing liver damage by increasing adipogenesis through upregulation of adipog and adiq gene expression and the leptin expression gene lep [28]. In addition, fucoxanthin and fucoidan activate the SIRIPGC-1 axis in the liver, which is an important pathway in hepatic lipid metabolism by improving mitochondrial function and fatty acid oxidation [28]. Activation of the SIRI-PGC-1 axis can reduce liver injury that can cause liver fibrosis [28].
In another study, fucoxanthin could reduce triglyceride and cholesterol levels in the liver, which is characterized by decreased mRNA expression of lipogenic enzymes such as acetyl CoA carboxylase (ACC), fatty acid synthase (FAS), and G6PDH which then reduces lipid accumulation in the liver [21]. In this process, there is also a decrease in the transcriptional factor SREBP1-c, which influences insulin function and activation of fatty acid and cholesterol biosynthetic pathways [21]. Fucoxanthin also increases the excretion of TG and cholesterol through feces; this activity is influenced by the inhibition of pancreatic lipase activity that triggers fat and cholesterol malabsorption [21].
Several studies have also shown the effect of consuming Chlorella vulgaris to reduce serum cholesterol in hyperlipidemic patients and mild hypercholesterolemia by inhibiting the absorption of dietary and endogenous lipids [37]. The use of Chlorella vulgaris has been widely studied because of its effect on lipid metabolism, which results in a decrease in the concentration of TG that accumulates in the liver while increasing insulin sensitivity, which contributes to the storage of TG in adipose tissue [38]. Chlorella vulgaris can also increase the excretion of cholesterol in the feces due to the presence of dietary fiber, which can increase the pool size of bile acids and excretion of fecal steroids through up-regulation of cholesterol 7-hydroxylase (CYP7A1), which can increase the cholesterol-lowering effect, which also inhibits the activity of bile acid absorption in the intestine [38, 39].
The combination of Chlorella vulgaris with metformin and vitamin E can increase the improvement of liver injury. Many reviews discuss the effect of vitamin E on improving liver enzymes as well as the histologic abnormalities present in NAFLD, in which the combination of vitamin E with lifestyle interventions can provide a better effect [40-42]. Vitamin E can act as an antioxidant that plays an important role in reducing liver injury due to inflammatory activity induced by lipid accumulation [43, 44]. Several studies on metformin have also demonstrated effects on improving biochemical and metabolic parameters in NAFLD patients [45]. Metformin benefits by increasing insulin sensitivity, which can induce a decrease in total body fat and visceral fat [46]. From this review, the addition of vitamin E and metformin with supplementation of Chlorella vulgaris can enhance the improvement of liver injury in NAFLD.
This review has several limitations. One of them is the inclusion criterion which only includes Englishlanguage studies. By limiting only the findings to English, it can limit the generalization of population conditions in the real world. However, this condition may be due to the use of English commonly used in the publication of existing studies. In addition, this review did not address the histologic outcome of liver biopsy, which is the gold standard for the diagnosis of NAFLD, although the use of liver enzymes to assess liver injury in NAFLD may help in screening and identification of patients with metabolic risk factors [31, 47]. Larger studies with longer follow-up times in more heterogeneous populations are needed to better assess the quality of the benefits of seaweed supplementation as well as to provide additional information regarding clinical outcomes and possible side effects.
Conclusions
Seaweed supplementation can improve liver injury as well as producing good clinical outcomes related to metabolic factors in NAFLD patients. Further studies in the future on a wider and heterogeneous population are needed to determine the effectiveness and safety of seaweed supplementation for NAFLD patients.