Congenitally corrected transposition of the great arteries (ccTGA) accounts for less than 1% of all congenital cardiac anomalies [1]. Unlike traditional transposition, ccTGA features both atrioventricular and ventriculoarterial discordance, allowing relatively normal circulatory function despite abnormal connections. CcTGA often coexists with associated defects, such as ventricular septal defect (VSD), pulmonary stenosis or atresia, tricuspid valve dysplasia, right ventricle hypoplasia or conduction issues, impacting prognosis [2]. Palliative procedures such as the Blalock-Taussig shunt (BTS) or pulmonary artery banding are commonly performed to address hypoxemia and circulatory failure in infancy. Definitive repair may involve technically demanding and high-risk double switch [3]. This report describes a patient with ccTGA {S,L,L}, dextrocardia, pulmonary valve atresia, and a large VSD who underwent staged interventions, including a BTS and a hemi-Mustard-Rastelli-Glenn procedure. We discuss the diagnostic challenges, surgical approach, postoperative care, and follow-up.
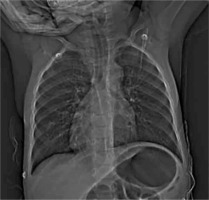
A male neonate was transferred to our hospital shortly after birth due to cyanosis and desaturation, suggesting a complex congenital heart defect. Born at 38 weeks via caesarean section with a birth weight of 3.1 kg, he had Apgar scores of 10/10/10 at 1, 3, and 5 minutes. The heart defect was first suspected postnatally following hypoxemia. Upon arrival, the patient showed respiratory distress with oxygen saturation at 80%, a heart rate of 160 bpm, and a respiratory rate of 60 breaths per minute. Physical examination revealed a 3–4-second capillary refill time, mild cyanosis, a 3–4/6 systolic murmur along the left sternal border, and hepatomegaly. Peripheral pulses were weak. Prostaglandin E1 infusion was initiated to maintain ductal patency and pulmonary blood flow. Broad-spectrum antibiotics were started due to petechiae and elevated inflammatory markers, raising concern for congenital infection. Nasal continuous positive airway pressure (nCPAP) was required for respiratory support. Chest X-ray revealed dextrocardia, a cardiothoracic index of 0.52, and hyperinflation of the lungs, with no infiltrative changes (Figure 1).
Figure 1
Chest X-ray image of the patient before surgical treatment revealing dextrocardia, heart enlargement, and hyperinflated lungs
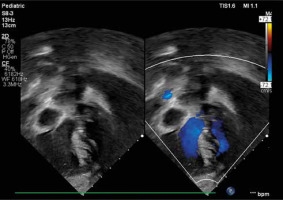
Bedside echocardiography revealed {S,L,L} dextrocardia, situs solitus, and discordant atrioventricular and ventriculoarterial connections (ccTGA). Pulmonary valve atresia was noted, with a valve annulus measuring 5 mm and a hypoplastic pulmonary trunk measuring 6.2 mm. The right ventricle was hypertrophied and slightly hypoplastic (Figure 2). A large, non-restrictive ventricular septal defect (VSD) measured 7 × 9 mm with left-to-right shunting. A 5.5 mm atrial septal defect (ASD) showed bidirectional shunting and a tortuous patent ductus arteriosus (PDA) exhibited significant left-to-right flow. Pulmonary arteries measured 3.5 to 4 mm bilaterally. The aorta arose from the right ventricle, positioned anterior and left of the hypoplastic pulmonary trunk.
Figure 2
Echocardiography showing {S,L,L} TGA with left ventricle on the right side and left-sided, hypertrophied, slightly hypoplastic right ventricle
These findings confirmed ccTGA with dextrocardia, pulmonary valve atresia, a large VSD, and a PDA, explaining the patient’s severe cyanosis and reliance on the PDA for pulmonary circulation.
Given the clinical presentation and anatomy, the patient underwent a right Blalock-Taussig shunt (RBTS) to increase pulmonary blood flow. The surgery, performed via midline sternotomy under general anaesthesia, involved implanting a 4 mm Gore-Tex graft between the right pulmonary artery and brachiocephalic trunk and closing the arterial duct.
The early postoperative course, with a 4.5 μg/kg/min dopamine infusion, was complicated by failed extubation and pericardial effusion. After a successful second extubation, serial echocardiograms confirmed a patent Blalock-Taussig shunt with a 4 mm Gore-Tex graft and 28–36 mm Hg gradient. Mild stenosis in both pulmonary arteries was noted, but oxygenation remained adequate, and ventricular function was normal.
The child showed steady growth during the follow-up after RBTS, with oxygen saturations ranging from 80% to 85%. Later, they declined slightly to 75–80% due to the expected decrease in shunt effectiveness as the child grew. Regular assessments were conducted to monitor shunt patency, pulmonary artery development, and overall cardiac function.
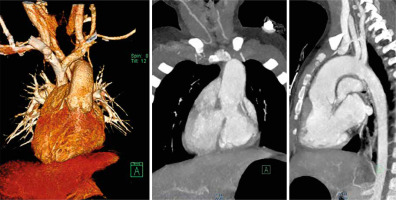
At the age of 2 years, the patient was admitted for definitive surgical correction to address both the complex heart anatomy and progressive cyanosis. A thorough preoperative evaluation was conducted, including echocardiography, angio-CT, and cardiac catheterization. Echocardiography confirmed dextrocardia and ccTGA, with the right ventricle connected to the systemic circulation and the left ventricle to the pulmonary circulation. The Blalock-Taussig shunt was patent, though mild stenosis was noted in the right pulmonary artery (RPA). Both pulmonary arteries were mildly underdeveloped, with the RPA measuring 8 mm and the left pulmonary artery (LPA) 5.5–6.5 mm. Right ventricular hypertrophy, slight hypoplasia and mild RV systolic dysfunction were observed, with an ejection fraction of 64%. Flow through the superior vena cava (SVC) and pulmonary veins was normal. Computed tomography imaging showed the pulmonary trunk in the aortic position and the aorta anterior to it. The pulmonary trunk measured 8 mm, showing mild narrowing, while the left and right pulmonary arteries measured 11 mm and 9 mm, respectively, with normal flow. The aorta was normal in size but abnormally positioned. Coronary arteries had standard origins, and the pulmonary venous connection was normal (Figure 3).
Figure 3
Computed tomography angiography with 3D reconstruction, AP projection and lateral view of the corrected transposition of the great arteries
Cardiac catheterization revealed moderately elevated pulmonary artery pressures and increased LPA resistance (narrowest at 5.5 mm). Pulmonary vascular resistance (PVR) was within the limits for proceeding with the planned surgery.
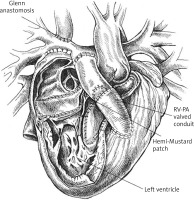
The patient underwent the hemi-Mustard-Rastelli-Glenn procedure through a midline sternotomy. After confirming dextrocardia, the right and left pulmonary arteries, brachiocephalic trunk and RBTS were dissected. The cardio-pulmonary bypass was initiated, and when the body temperature reached 32°C, DelNido blood cardioplegia was administered. The right atrium was opened, the atrial septum excised, and the opening of the coronary sinus was widened. A patch (Photophix) was implanted to redirect blood flow from the inferior vena cava (IVC) through the interatrial septum to the tricuspid valve, separating systemic from pulmonary venous blood. A large incision was made in the right ventricular outflow tract, and a Gore-Tex patch was used to tunnel blood from the left ventricle through the VSD to the aorta, ensuring proper systemic circulation. The superior vena cava (SVC) was disconnected from the right atrium and anastomosed to the right pulmonary artery, enabling passive venous blood flow to the lungs. A 21 mm Gore-Tex conduit was used to reconstruct the right ventricular outflow tract, and a 19 mm Trifecta valve was implanted. The pulmonary trunk was closed off (Figure 4). The heart was re-perfused, sinus rhythm restored, and pacing leads placed. The patient was weaned off bypass with inotropic support (milrinone, epinephrine, dopamine). Postoperative monitoring in the pediatric cardiac ICU focused on oxygenation and hemodynamic stability. The patient was extubated on day two and gradually weaned off inotropes, maintaining a stable sinus rhythm.
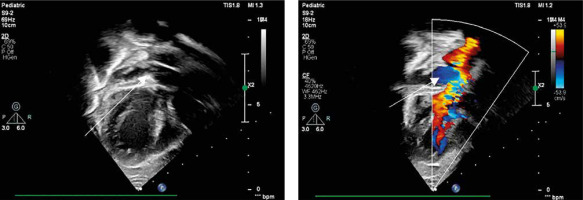
Postoperative echocardiography showed good biventricular function, a 64% ejection fraction, no significant residual defects, and a patent Glenn anastomosis. The Trifecta valve functioned well, with minimal regurgitation. Pulmonary arteries measured 8.5 mm (right) and 6.5 mm (left) proximally (Figure 5). The chest X-ray showed a slightly enlarged heart due to right ventricular hypertrophy, but no pleural effusions or pneumothorax.
Figure 5
Echocardiography showing flow from inferior vena cava through hemi-Mustard tunnel and interatrial septum to the left atrium
The patient was discharged in good condition with a regimen including furosemide, warfarin, and endocarditis prophylaxis. Regular follow-up in cardiology and cardiac surgery clinics was scheduled, along with routine pediatric care. Over the next 2 years, follow-up focused on monitoring the function of the Glenn shunt, RV-PA conduit, and Trifecta valve, and the growth of the pulmonary arteries.
The most recent echocardiography in July 2024 showed stable biventricular function with no systemic or pulmonary venous flow stenosis. The right ventricular hypertrophy had decreased. The Glenn anastomosis and Gore-Tex conduit continued to function well, with stable flow velocities and no significant stenosis or obstruction.
The patient’s growth and neurodevelopment regarding overall health and development have been age-appropriate. He engages in mild physical activities and experiences no significant limitations in daily life. Periodic assessments indicated no developmental delays or surgery-related complications.
The paper reports the first successful hemi-Mustard-Rastelli-Glenn surgery in Poland in a child with {S,L,L} TGA, dextrocardia and pulmonary valve atresia with intermediate-term follow-up. A combination of palliative and definitive surgical procedures, including the RBTS and corrective surgery, proved to be an effective strategy for managing the patient’s condition.
The hemi-Mustard-Rastelli-Glenn procedure offers a robust solution for patients with ccTGA and additional anomalies such as left ventricular outflow tract obstruction or pulmonary stenosis/atresia, tricuspid valve abnormalities and positional heart anomalies. The Glenn anastomosis reduces the volume load on the failing right ventricle and reduces strain on a dysplastic tricuspid valve. It can be especially advantageous for patients with an RV-pulmonary artery conduit. In the presented patient, pulmonary atresia and hypoplastic pulmonary arteries made this combination approach especially beneficial, addressing systemic-to-pulmonary blood flow and discordant ventricular connections. The hemi-Mustard- and hemi-Senning-Rastelli-Glenn procedures are considered variants of the double switch operation [4, 5]. Compared to classic atrial switch procedures such as the Senning or Mustard operations, the hemi-Mustard or hemi-Senning approach has fewer long-term complications, including venous pathway stenosis, particularly in cases with positional anomalies and a reduced grade of right ventricular dysfunction. Some centers recommend the procedure as a primary choice for the whole spectrum of ccTGA variants, with excellent long-term results [6].
Studies on patients undergoing the hemi-Mustard-Rastelli-Glenn procedure have shown outstanding results, low mortality rates and good functional performance. Most patients with preserved biventricular function maintain NYHA class I or II status. Conduit-related complications, such as narrowing or dysfunction, can typically be managed with later interventions such as interventional cardiology methods or conduit replacement. In this case, early and mid-term results are consistent with the literature, as the patient showed satisfactory conduit function and good ventricular contractility postoperatively [7].
Clinical studies indicate that patients who undergo the hemi-Mustard-Rastelli-Glenn or hemi-Senning-Rastelli-Glenn procedure have better mid-term outcomes, including survival rates, venous pathways obstruction, conduit longevity (lower flow through RV because of Glenn anastomosis) or sinus node dysfunction compared to those who undergo the literal double-switch [5, 8, 9]. The absence of sinus node dysfunction or systemic/pulmonary venous drainage obstruction in our patient further emphasizes the benefits of the hemi-Mustard approach. Moreover, because only blood from the inferior vena cava flows through the valve, the size of the valve (19 mm) and the conduit (20 mm) ensure long-term function without the need for re-intervention.
The published results clearly suggest that successful outcomes after the hemi-Mustard-Rastelli-Glenn procedure depend on early intervention, careful management of pulmonary circulation, systemic ventricle function and prevention of right ventricular deterioration. Moreover, it has been shown that patients who do not qualify for double switch surgery due to systemic or pulmonary ventricular dysfunction can successfully undergo the hemi-Senning- or hemi-Mustard-Rastelli-Glenn procedure. The postoperative condition of our patient with mild pulmonary artery narrowing and trace regurgitation through the Trifecta valve is consistent with the literature, showing that these issues are manageable and do not significantly impact the long-term prognosis.
While early and mid-term outcomes are favorable, long-term monitoring is crucial, particularly for conduit dysfunction, ventricular function, and potential arrhythmias. Studies suggest that even with good initial outcomes, continued follow-up is essential to detect late complications, such as conduit replacement or arrhythmia management [10].
The hemi-Mustard-Rastelli-Glenn procedure provided a durable solution for ccTGA with pulmonary atresia, VSD and dextrocardia. Mid-term outcomes are excellent compared to similar patients. Thorough follow-up is necessary to ensure a continued good clinical condition and address arising conduit-related problems.