Introduction
Epidemiological observations from the last decades demonstrate a rise in the incidence of allergic rhinitis and asthma in developed countries [1]. They are currently diseases with major prevalence and morbidity rates for people under 30 years old suffering from non-infectious chronic diseases [2–5]. Numerous studies were performed as part of the Epidemiology of Allergic Diseases in Poland (ECAP) survey, proving the epidemiological significance of these diseases and great diversity of allergy risk factors [6–9]. Determination of specific IgE in respondents’ serum, a reliable method to evaluate allergic hypersensitivity [10, 11], supplements the results of this survey [12].
Aim
The aim of the study was to determine the relationship between the concentration of specific IgE antibodies in serum and types of rhinitis.
Material and methods
The quantitative data presented in the article were collected as part of the Epidemiology of Allergic Diseases in Poland (ECAP) project and its continuation. The ECAP comprised 2 main phases: (i) a questionnaire-based study (computer-assisted personal interview – CAPI); and (ii) a complementary clinical assessment (spirometry with bronchodilator challenge, skin-prick tests, peak nasal inspiratory flow, and blood sampling for genetic and immune tests). A total of 18,617 individuals from 8 cities (each with a population in excess of 150,000) and one rural region took part in the study (phase one). The sample was drawn (by stratified cluster sampling method) from a personal identity number (PESEL) database (maintained by the Minister of the Interior and Administration). 4783 respondents were randomly selected and examined by allergists (phase 2 of the study). Blood from 4077 respondents was collected, and the concentration of sIgE antibodies against allergens d1 (Dermatophagoides pteronyssinus), e1 (cat dander), g6 (timothy grass), and m6 (Alternaria alternata) was determined in serum, using the reference method CAP (Phadia reagents, UniCAP 100 laboratory system). A concentration of sIgE antibodies of at least 0.35 IU/ml (classes 1–6) or 0.7 IU/ml (classes 2–6) was considered positive. The sIgE-determined respondents included 2223 females and 1854 males. 1026 respondents were aged 6–7 years, 1153 respondents were aged 13–14 years, and 1898 respondents were adults. An exact methodology of the ECAP survey is described at www.ecap.pl [12] and in the "Polish Journal of Allergology" [13].
The results of determination of sIgE antibodies were correlated as follows:
to the following clinical diagnoses: healthy , intermittent allergic rhinitis, persistent allergic rhinitis, nonallergic rhinitis, seasonal allergic rhinitis, perennial allergic rhinitis, nasal polyps;
to results of skin-prick tests: negative (0–2 mm), + (3–5 mm), ++ (6–8 mm), +++ (at least 9 mm).
Statistical analysis
The aim of the statistical analysis was to compare proportions of people with a high level of immunoglobulin in 2 groups. The classical approximate test for comparison of 2 proportions was applied [14]. If the calculated p-value was less than 0.05, a statistically significant difference between the investigated proportions was recognised. Otherwise, the fractions of people with a high level of immunoglobulin in the investigated groups was treated as similar. Calculations were performed using the statistical package Statistica (Statistica, Tulsa, Oklahoma, US).
Results
In respondents with allergic rhinitis, sIgE antibodies against D. pteronyssinus and timothy grass were the most frequently detected (“D. pteronyssinus” vs. “cat dander”, classes 1–6 p < 0.001, classes 2–6 p < 0.001; “timothy grass” vs. “cat dander”, classes 1–6 p < 0.001, classes 2–6 p < 0.001). In the same group, sIgE antibodies against A. alternata were the least frequently detected (“A. alternata” vs. “cat dander”, classes 1–6 p < 0.001, classes 2–6 p < 0.005) (Table 1).
Table 1
Number (percentage) of respondents with sIgE concentration ≥ 0.35 IU/ml (classes 1–6) or ≥ 0.7 IU/ml (classes 2–6) – respondents with allergic rhinitis
sIgE antibodies against any allergen were detected in 60.1% (classes 1–6)/53.3% (classes 2–6) of respondents with allergic rhinitis, but also in 9.9% (classes 1–6)/7.6% (classes 2–6) of healthy respondents.
sIgE antibodies against D. pteronyssinus were more frequently detected in respondents with persistent allergic rhinitis than in respondents with intermittent allergic rhinitis (classes 1–6 p < 0.001, classes 2–6 p < 0.001), relating to sIgE antibodies against cat dander, this disparity is lower (classes 1–6 p < 0.1, classes 2–6 p < 0.1), relating to sIgE antibodies against A. alternata, no statistically significant differences were identified. sIgE antibodies against timothy grass were more frequently detected in respondents with intermittent allergic rhinitis than in respondents with persistent allergic rhinitis (classes 1–6 p < 0.05, classes 2–6 p < 0.05) (Table 2).
Table 2
Number (percentage) of respondents with sIgE concentration ≥ 0.35 IU/ml (classes 1–6) or ≥ 0.7 IU/ml (classes 2–6) – respondents with intermittent, persistent, seasonal, or perennial allergic rhinitis
sIgE antibodies against D. pteronyssinus were more frequently detected in respondents with perennial allergic rhinitis than in respondents with seasonal allergic rhinitis (classes 1–6 p < 0.001, classes 2–6 p < 0.001). sIgE antibodies against timothy grass were more frequently detected in respondents with seasonal allergic rhinitis than in respondents with perennial allergic rhinitis (classes 1–6 p < 0.001, classes 2–6 p < 0.001). Relating to allergens of cat dander and A. alternata, no statistically significant differences were identified (Table 2).
Relating to an allergen of timothy grass, sIgE antibodies were more frequently detected in respondents with intermittent allergic rhinitis than in healthy respondents, regardless of skin-prick test (negative, ++, +++ p < 0.005 to p < 0.001). Relating to allergens of D. pteronyssinus, cat dander, and A. alternata, sIgE antibodies were more frequently detected in respondents with intermittent allergic rhinitis and a negative skin-prick test as compared to healthy respondents with a negative skin-prick test (classes 1–6, D. pteronyssinus p < 0.005; classes 2–6 p < 0.005 to p < 0.001) (Table 3, Figure 1).
Table 3
Number (percentage) of respondents with sIgE concentration ≥ 0.35 IU/ml (classes 1–6) or ≥ 0.7 IU/ml (classes 2–6) – healthy respondents and respondents with intermittent or persistent allergic rhinitis
Figure 1
Percentage of respondents with sIgE concentration ≥ 0.35 IU/ml (classes 1–6) or ≥ 0.7 IU/ml (classes 2–6) – healthy respondents and respondents with intermittent or seasonal allergic rhinitis
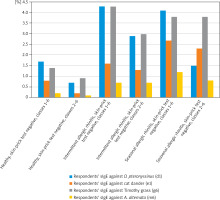
Relating to allergens of D. pteronyssinus, cat dander, and A. alternata, sIgE antibodies were more frequently detected in respondents with persistent allergic rhinitis and a negative skin-prick test as compared to healthy respondents with a negative skin-prick test. Numerous statistically significant differences were identified (p < 0.005 to p < 0.001). Relating to allergens of D. pteronyssinus and cat dander, sIgE antibodies were more frequently detected in respondents with persistent allergic rhinitis and a weakly positive skin-prick test as compared to healthy respondents with a weakly positive skin-prick test. Numerous statistically significant differences were identified (p < 0.05 to p < 0.001). Relating to an allergen of timothy grass, sIgE antibodies were more frequently detected in respondents with persistent allergic rhinitis and a positive skin-prick test as compared to healthy respondents with a positive skin-prick test. Numerous statistically significant differences were identified (p < 0.05 to p < 0.001) (Table 3, Figure 2).
Figure 2
Percentage of respondents with sIgE concentration ≥ 0.35 IU/ml (classes 1–6) or ≥ 0.7 IU/ml (classes 2–6) – healthy respondents and respondents with persistent or perennial allergic rhinitis
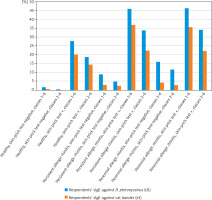
Relating to an allergen of timothy grass, sIgE antibodies were more frequently detected in respondents with seasonal allergic rhinitis than in healthy respondents, regardless of skin-prick test result (negative, ++, +++ p < 0.05 to p < 0.001). Relating to allergens of D. pteronyssinus, cat dander, and A. alternata, sIgE antibodies were more frequently detected in respondents with seasonal allergic rhinitis and a negative skin-prick test as compared to healthy respondents with a negative skin-prick test result. Numerous statistically significant differences were identified (p < 0.05 to p < 0.005). Relating to an allergen of cat dander, sIgE antibodies were more frequently detected in respondents with seasonal allergic rhinitis and a weakly positive skin-prick test as compared to healthy respondents with a weakly positive skin-prick test (classes 1–6 p < 0.005, classes 2–6 p < 0.05) (Table 4, Figure 1). Relating to allergens of D. pteronyssinus and cat dander, sIgE antibodies were more frequently detected in respondents with perennial allergic rhinitis and a negative skin-prick test as compared to healthy respondents with a negative skin-prick test (p < 0.001) and in respondents with perennial allergic rhinitis and a weakly positive skin-prick test as compared to healthy respondents with a weakly positive skin-prick test (p < 0.05). Relating to an allergen of timothy grass, sIgE antibodies were more frequently detected in respondents with perennial allergic rhinitis and a medium or strongly positive skin-prick test as compared to healthy respondents with a medium or strongly positive skin-prick test result. Numerous statistically significant differences were identified (p < 0.05 to p < 0.005) (Table 4, Figure 2).
Table 4
Number (percentage) of respondents with sIgE concentration ≥ 0.35 IU/ml (classes 1–6) or ≥ 0.7 IU/ml (classes 2–6) – healthy respondents and respondents with seasonal or perennial allergic rhinitis
Discussion
Numerous studies were performed as part of the ECAP survey, proving the epidemiological significance of these diseases and great diversity of allergy risk factors. Determination of specific IgE in serum of the respondents, the most reliable method to evaluate allergic hypersensitivity, has been the continuation of ECAP.
sIgE antibodies against D. pteronyssinus were more frequently detected in respondents with persistent allergic rhinitis than in respondents with intermittent allergic rhinitis. Relating to IgE antibodies against cat dander, this disparity is lower. Relating to sIgE antibodies against A. alternata, no statistically significant differences were identified. sIgE antibodies against timothy grass were more frequently detected in respondents with intermittent allergic rhinitis than in respondents with persistent allergic rhinitis. In a study by Corsico et al., specific IgE levels to house-dust mite were significantly higher in patients with persistent allergic rhinitis than in patients with intermittent allergic rhinitis [15]. On the other hand, in a study by Bauchau et al., subjects with persistent allergic rhinitis were more often sensitized to grass pollen, but less often to house dust mites, than subjects with intermittent allergic rhinitis [16]. Furthermore, in a study by Bousquet et al., most patients with intermittent allergic rhinitis had a pollen sensitivity, but 5% had a single house dust mite sensitization, and over 50% of patients with persistent allergic rhinitis were sensitized to pollens or house dust mites [17]. sIgE antibodies against D. pteronyssinus were more frequently detected in respondents with perennial allergic rhinitis than in respondents with seasonal allergic rhinitis. sIgE antibodies against timothy grass were more frequently detected in respondents with seasonal allergic rhinitis than in respondents with perennial allergic rhinitis. Relating to allergens of cat dander and A. alternata, no statistically significant differences were identified. In a study by Stoltz et al., sensitization to seasonal compared with perennial allergens was more closely associated with rhinitis risk [18]. In a study by Rolinck-Werninghaus et al., baseline specific IgE, but not total IgE, was associated with symptom severity during the pollen season in children with seasonal allergic rhinitis [19]. In a study by Hatzler et al., testing IgE sensitization at a preclinical stage facilitated prediction of seasonal allergic rhinitis [20]. On the other hand, in a study by Nickelsen et al., there was a lack of correlation between titres of serum or nasal secretory grass-specific IgE and symptoms in untreated patients with seasonal allergic rhinitis [21]. In the study reported in this article, sensitization to D. pteronyssinus increased the probability of persistent/perennial allergic rhinitis, and sensitization to timothy grass increased the probability of intermittent/seasonal allergic rhinitis. The lack of correlation between sensitization to cat dander and persistent/perennial allergic rhinitis presumably resulted from a seasonally variable indoor concentration of animal hair. Allergens of A. alternata are present indoors all year long, but their concentration fluctuates in particular months, which probably causes the lack of correlation between sensitization to A. alternata and persistent/perennial allergic rhinitis or intermittent/seasonal allergic rhinitis.
When sIgE antibodies against timothy grass were detected, the occurrence of intermittent/seasonal allergic rhinitis is much more probable. Relating to respondents with a negative skin-prick test with allergens of D. pteronyssinus, cat dander, or A. alternata, when sIgE antibodies against the same allergen were detected, the occurrence of intermittent/seasonal allergic rhinitis is much more probable. Therefore, it is worth determining specific IgE antibodies in patients with symptoms of intermittent or seasonal allergic rhinitis and negative skin-prick tests. The results of this determination enhance the likelihood of an accurate diagnosis.
Relating to respondents with a negative or weakly positive skin-prick test with allergens of D. pteronyssinus or cat dander, when sIgE antibodies against the same allergen were detected, occurrence of persistent/perennial allergic rhinitis was much more probable. Relating to respondents with a medium or strongly positive skin-prick test with an allergen of timothy grass, when sIgE antibodies against the same allergen were detected, occurrence of persistent/perennial allergic rhinitis was much more probable. Thus, it is worth determining specific IgE antibodies in patients with symptoms of persistent or perennial allergic rhinitis and a negative or weakly positive skin-prick test with allergens of D. pteronyssinus or cat dander. The results of this determination enhance the likelihood of an accurate diagnosis, as previously.
Conclusions
sIgE antibodies against any allergen are detected in 60.1% (classes 1–6)/53.3% (classes 2–6) of respondents with allergic rhinitis, but also in 9.9% (classes 1–6)/7.6% (classes 2–6) of healthy respondents. Relating to respondents with a negative skin-prick test, when sIgE antibodies against the same allergen are detected, occurrence of intermittent/seasonal allergic rhinitis is much more probable. Relating to respondents with a negative or weakly positive skin-prick test with allergens of D. pteronyssinus or cat dander, when sIgE antibodies against the same allergen are detected, occurrence of persistent/perennial allergic rhinitis is much more probable.








