Introduction
Basal cell carcinoma (BCC) is the most common skin cancer in humans, occurring in more than 50% of Caucasians during their lifetime, with a frequency rate that is continually increasing [1–3]. Although BCC rarely metastasizes, it can cause significant morbidity if left without medical care [4, 5]. Its prevalence is related to increasing age. Most of BCCs develop in the facial area, and progression of the tumour may cause significant tissue destruction leading to major disfigurement [4, 6]. That is why it is essential to find prognostic factors or markers that can identify BCC at an early stage or help to understand the oncogenesis of this most common cancer.
There are reports identifying some proteins playing a role in BCC pathogenesis. In this spectrum of different representatives, the role of transforming growth factor β (TGF-β) and antimicrobial peptides (AMPs) is emphasized.
Transforming growth factor β is a family of multifunctional regulatory peptides that participate in immunomodulation, morphogenesis, and wound healing; they also play a role in regulating cell growth and differentiation [7]. Additionally, the effect of TGF-β expression is described in different cancers; however, the results are inconsistent because this peptide can have the effect of a tumour suppressor or stimulate its growth depending on the stage of neoplasm [8]. Antimicrobial peptides are one of the primary mechanisms used by the skin in the early stages of immune defence [9]. The family of AMPs is quite broad, but it mainly includes defensins, cathelicidin, dermcidin, and psoriasin [10]. Human β-defensins (HBDs) can be up- or downregulated depending on the specific cancer type and anatomic localization [11]. Like in TGF-β, the expression of defensins and cathelicidin can change with neoplasia development. Additionally, the literature regarding TGF-β, cathelicidin, and HBD levels in BCCs is inconsistent and lacking data necessary to establish future diagnostic and therapeutic features of those peptides. Therefore, a systemic review concerning the role of the proteins mentioned above in BCC is needed.
Aim
We present a systematic review summarizing the role of TGF-β, cathelicidin, and HBDs in the pathogenesis of BCC.
Material and methods
A systematic review of the literature was performed and reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement.
The major online databases including PubMed, Scopus, Embase, Web of Science, Cochrane Library, and Google Scholar were searched to extract studies regarding the levels of TGF-β, HBD, and cathelicidin in BCC. In addition, studies lacking numerical data with descriptive results were also requested. The following search terms, established in compliance with Booleans logic, were used: ((bcc) OR (basal cell carcinoma)) AND ((TGF) OR (transforming growth factor) OR (defensin) OR (CAMP) OR (Cathelicidin antimicrobial peptide)). Date, language, article type, and/or text availability conditions were not applied. An additional search was done at the end through the references of the included studies.
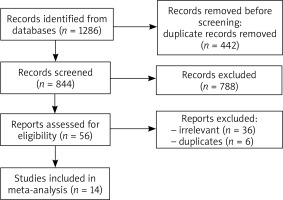
The search lasted from November 2021 to January 2022. In the beginning, a total of 1286 studies were initially evaluated by 2 independent reviewers. Of those, 56 were adequate for a full text evaluation. Outcomes that were expected to be established were as follows: (1) levels of TGF-β, HBD’s, and cathelicidin in BCCs, (2) the influence of each protein on the proliferation rate of the BCCs, (3) determination of whether each of the proteins should be considered as a risk factor. The inclusion criteria included original studies with any quantitative or qualitative data regarding the presence and/or level of at least one of the concerned proteins, whereas the exclusion criteria involved case reports, conference reports, case series, review, letters to the editor, and irrelevant studies. Thirty-six studies were excluded during the full text evaluation due to irrelevance, and 6 were duplicated.
Finally, a total of 14 studies were included in this systematic review. To establish the risk of bias of each submitted study, the QUADAS-2 tool was used. The process of collecting the data is shown in Figure 1.
Results
A total of 14 studies met the inclusion criteria and were included in this systematic review [1, 2, 5–9, 12–18]. Each of the 14 submitted studies was deeply analysed. The studies included in this systematic review were not burdened and did not differ substantially in the risk of potential bias.
There were 6 studies that included initially established levels of TGF-β in BCCs. A total of 87 BCCs were analysed, and a common result was that the TGF-β levels increased in the BCCs compared to the control groups. Regrettably, they differed one from another greatly in methodology and in the way of presenting the gathered data. Therefore, authors decided not to establish new, overall numerical outcomes arising from those studies.
Analogously, 2 studies contained numerical data on HBD levels from different in methodologies. A total of 130 BCCs were analysed, and a common result was that HBD-2 levels or/and its mRNA expression were increased in the BCCs compared to the control groups. Furthermore, the level of mRNA expression of HBD-1 and HBD-2 was enormously different between lesional and non-lesional BCCs.
The level of cathelicidin was established in 108 BCCs and was significantly higher in the BCC group than in the control group. For a summary and brief description of each study containing these data, see Table 1. However, all studies included a comprehensive description of how and/or when each protein and/or associated receptors and/or genes affects BCCs and/or its role in BCCs pathogenesis.
Table 1
Characteristics of the submitted studies
Discussion
The aim of this paper was to systematically review the evidence of the role of TGF-β, HBDs, and cathelicidin in BCC and investigate whether these proteins can be a marker of BCC or a therapeutic target in the treatment of BCC.
Transforming growth factor β represents an evolutionarily conserved family of secreted polypeptide factors that regulate many aspects of physiological embryogenesis and adult tissue homeostasis [19]. It plays important roles in many biological processes, including cell growth, differentiation, apoptosis, migration, as well as cancer initiation and progression [20]. Dysregulation of TGF-β responsiveness and its downstream signalling pathways contribute to many diseases, including cancer initiation, progression, and metastasis [21]. In healthy cells and early-stage cancer cells, this pathway has tumour-suppressor functions, including cell-cycle arrest and apoptosis [22]. During the early stages of tumourigenesis, TGF-β acts as a tumour suppressor by inducing cytostasis and the apoptosis of normal and premalignant cells [23]. However, at later stages, when cancer cells have acquired oncogenic mutations and/or have lost tumour suppressor gene function, cells are resistant to TGF-β-induced growth arrest, and TGF-β functions as a tumour promotor by stimulating tumour cells to undergo the so-called epithelial-mesenchymal transition [23]. Statistically significant increases in the expression of TGF-β were detected in skin biopsies with diagnosed nodular BCC compared with the control group, confirming the important role of these proteins in skin carcinogenesis [24]. The dual function and pleiotropic nature of TGF-β signalling make it a challenging target and imply the need for careful therapeutic doses of TGF-β drugs and patient selection [22]. Recent studies have also suggested that the cross-talk between TGF-β signalling and other signalling pathways, such as Hippo, Wnt, EGFR/RAS, and PI3K/AKT pathways, may substantially contribute to our current understanding of TGF-β signalling and cancer [25]. As a result of the wide-ranging effects of TGF-β, blockade of TGF-β and its downstream signalling components provides multiple therapeutic opportunities [25]. Oncogenic transformation alters lipid metabolism to support tumour growth [24]. Gabitova-Cornell et al. in 2020 presented evidence from patient samples suggesting that activation of TGF-β signalling and epithelial-mesenchymal transition by cholesterol-lowering statins may promote the basal type of pancreatic ductal adenocarcinoma, conferring poor outcomes in patients [24]. It may be hypothesized that analogously, in patients with BCC, TGF-β signalling could be activated by cholesterol-lowering statins and, as a result, have an impact on BCC proliferation. This should be established in further studies. TGF-β1 is also considered as a crucial mediator in tissue fibrosis and causes tissue scarring largely by activating its downstream small mother against decapentaplegic signalling [26]. Additionally, TGF-β status may serve as a biomarker to predict responsiveness and as a therapeutic target to increase the activity of immunotherapies [27]. To summarize, TGF-β should be considered as an important factor in the pathogenesis of carcinomas such as BCC, and in the future as the potential therapeutic molecule.
Stamp et al. presented data on the role of TGF-β in patients with BCC in 1993. They gathered a group of 50 patients with different types of BCC and used the polyclonal antibody that recognizes TGF-β [18]. Their results were shown in a grading scale of evaluation as follows: +++ (strong), ++ (moderate), + (weak), and – (negative). Unfortunately, this way of presenting the results of the study is highly subjective, and precise data cannot be analysed with other, more objective results. However, the article still outlines the path for further studies and signals a potential relationship between the TGF-β level and BCC proliferation. The analysis revealed that TGF-β is present in the stroma surrounding BCC in the majority of cases (29 out of 50) [18]. The authors concluded that many of the characteristics of BCC could be explained by the effects of TGF-β [18]. Four years later, Furue et al. published a similar study based on a smaller group (6 patients with BCC and 8 healthy controls) but using polyclonal antibodies [7]. Their results were shown also in a grading score: ++ (strongly positive), + (positive), ± (weakly positive), – (negative) [7]. The authors also concluded that in most cases with BCC, the TGF-β was accumulated in stromal cells, suggesting that the secretion of collagen from the stroma can be enhanced by TGF-β in an autocrine way at certain stages of BCC development [7]. Recently, Florescu et al. presented a study in 53 patients with BCC and also used a polyclonal antibody [8]. Their intensity score was as follows: 1 – mild, 2 – moderate, and 3 – strong. Immunohistochemical analysis revealed the presence of TGF-β immunoreactions in most patients with BCC [8]. The authors described a positive linear correlation between TGF-β3 and TGF-βRIII in relation to the type of BCC and its progression. The presented data suggest that TGF-β is involved in the aggressiveness of BCC, and this can be transferred to the potential therapeutic targets in this common skin cancer [8].
In 2005 Adamek et al. presented an exciting study on changes in immune system activity by supplying photodynamic therapy in patients with BCC [16]. The group consisted of 17 patients with different types of BCC. The authors checked the level of TGF-β from blood samples with Elisa Kit before and after photodynamic therapy [16]. A statistically significant difference was shown in the level of TGF-β before and after treatment; after photodynamic treatment, a remarkable decrease in the level of TGF-β was observed [16].
Gambichler et al. in 2007 presented a study on the expression of TGF-β in patients with BCC (n = 24) in comparison to healthy controls (n = 25) [14]. Their analysis was based on real-time-PCR (RT-PCR), and it was conducted in 3 samples: one taken from the centre of BCC (lesional), the second from the adjacent healthy skin in BCC patients (non-lesional), and the third sample from heathy skin controls [14]. The authors revealed that mRNA levels of TGF-β observed in healthy skin from the control group did not differ from those of TGF-β detected in non-lesional skin in patients with BCC [14]. However, significant mRNA overexpression of TGF-β was revealed in a lesional sample of BCC compared with non-lesional skin but still from BCC patients [14]. The authors concluded that their data suggest a possible role of TGF-β in the pathogenesis of BCC [14]. Similar data were presented by Ciążyńska et al. [2]. The authors presented the activity of TGF-β based on 22 patients with BCC and 22 healthy controls using integrated density values evaluated by RT-PCR. The difference between groups was statistically significant; the density of TGF-β was significantly higher in patients with skin cancer than in healthy control [2]. Chu et al., similarly to the authors mentioned above, used RT-PCR to detect TGF-β in 49 patients diagnosed with BCC between 2004 and 2005 [15]. The authors revealed that TGF-β1 was intensely expressed in the invasive form of BCC and thus might serve as a surrogate marker for invasive BCC [8].
Human β-defensin-1 is a multifaceted antimicrobial peptide that is a tumour suppressor and, depending on the call of duty, is capable of inducing self-nets and neutrophil extracellular traps (NET) to capture and/or kill bacteria, and it participates in inflammatory responses in chronic diseases [28]. Studies described so far suggest that HBD-2 is an important protein of innate immune response, which provides protection for the human organism against invading pathogens of bacterial, viral, fungal, as well as parasitic origin [29]. The skin microbiota plays a prominent role in health and disease; however, its contribution to skin tumourigenesis is not well understood [30]. Madhusudhan et al. indicated that a changed microbial community composition in squamous cell carcinoma, specified by Staphylococcus aureus overabundance, might promote tumour cell growth via modulation of HBD-2 expression. The influence of HBD-2 on BCCs may be hypothesized, and this should be established in further studies. In addition to its antimicrobial activity, the skin-derived antimicrobial peptide HBD-3 promotes keratinocyte proliferation and migration to initiate the wound healing process; however, its effects on fibroblasts, which are the major cell type responsible for wound healing, remain unclear [31]. The significant correlation between HBD-3 mRNA expression and protein concentration suggests that mRNA expression could be used to predict protein levels [32].
Gambichler et al. also showed results concerning the presence of HBD1-3 mRNA by quantitative real-time RT-PCR in a group of 22 patients with BCC in comparison with 27 healthy controls. They observed a markedly altered expression of HBD-1 and HBD-2 in patients with BCC [5]. The authors revealed significantly decreased mRNA levels of HBD-1 in patients with BCC, indicating that this defensin is markedly downregulated in cancer tissue [5]. Furthermore, the authors demonstrated that HBD-2 levels are considerably up-regulated in patients with BCC [5]. The easiest method of checking the presence of HBDs in patients with BCC compared to healthy controls was presented by Fijałkowska et al. [4]. The authors checked the level of proteins in blood samples using an Elisa Kit. The data were based on a group of 108 people (49 patients with BCC and 59 healthy controls). The level of HBD-2 was significantly higher in patients with BCC than in healthy controls [4]. The level of HBD-1 was detected only in 12 patients (7 from the control group and 5 from BCC patients), which may support the theory explained by Gambichler et al. that HDB-1 is downregulated in cancer tissue. Therefore, it is not detected in patients with BCC and healthy controls, and it may be related to the presence of bacteria or viral agents [5].
LL-37, the C-terminal peptide of human cathelicidin antimicrobial peptide (CAMP, hCAP18), reportedly increases resistance to microbial invasion and exerts important physiological functions in chemotaxis, promotion of wound closure, and angiogenesis [33]. The findings that vitamin D-dependent induction of cathelicidin in human macrophages activates the anti-cancer activity of tumour-associated macrophages and enhances antibody-dependent cellular cytotoxicity support the critical roles of vitamin D-dependent induction of cathelicidin in cancer progression [33]. Elevated concentrations of cathelicidin are associated with the presence of BCC [9]. Additionally, the specificity of cathelicidin in the detection of BCC is high [9]. The influence and detailed mechanisms from which elevated cathelicidin levels in BCC arise should be established in further studies. Research on connections between BCC and cathelicidin may benefit because the specificity of cathelicidin in the detection of BCC may promote this peptide as a potential diagnostic and therapeutic molecule in the treatment of BCCs.
The main limitation of the presented review is the major heterogeneity among included studies, which also arrested the plan to perform a meta-analysis. One of the reasons of heterogeneity is the different methodologies used by authors in their research. Some of them were looking for the expression of proteins in tissues, and some were measuring the level of proteins in serum. That is why the obtained results were presented in a different way; just the presence of TGF-β or lack of TGF-β (±) or the serum level of TGF-β or HBDs or cathelicidin in pg/ml or ng/ml. The number of examined patients in analysed groups also vary, which is probably the result of the costs that must be paid, especially when performing examinations with polyclonal antibodies.
The literature regarding the role of TGF-β, cathelicidin, and HBDs in the pathogenesis of BCC is lacking biochemical mechanisms and the influence of those proteins on the proliferations of BCC, proven on larger cohort groups. The potential role and elevated levels of TGF-β, cathelicidin, and HBDs in BCCs are put in the spotlight. However, those reports, especially concerning the elevated levels of cathelicidin and HBDs, are relatively young. Therefore, a pathway in which further studies regarding BCC should be carried out comes to mind. Molecular and biochemical researchers should focus on providing the literature with proven explanations of mechanisms in which those proteins can affect the BCCs. This should deliver potential usage of TGF-β, cathelicidin, and HBDs for diagnostic and/or therapeutic features in the future. Additionally, further studies involving larger cohort groups should deliver more statistically significant standard levels of those proteins in patients with BCC. Vitamin D and cholesterol levels combined with those proteins in patients with BCC seems to be a promising research trend because it potentially implicates more diagnostic and/or therapeutic features in those cancers. Further studies should be standardized and present their results in the same way so that a combined, statistically significant conclusion could be established later on.
Conclusions
The presented review shows evidence that proteins like TGF-β, HBD, and cathelicidin play a role in developing BCC. Protein levels or their expression are elevated in patients with BCC. Further studies presenting comparable methodology are needed to strengthen the conclusion in a larger group of patients of different ethnicity to check whether, e.g., the simple blood sampling can measure the level of proteins constituting a marker in BCC, like BRCA in breast cancer or PSA in prostate cancer. Additionally, a critical review of the literature with an exposure of its deficiencies was presented and discussed.