Dear Editor,
Heparin-induced thrombocytopenia (HIT) is a prothrombotic disorder caused by antibodies against the platelet factor 4 (PF4)–heparin complex. HIT should be suspected in patients who are currently receiving heparin or who received heparin in the preceding 5 to 10 days and exhibit clinical symptoms including the new onset of thrombocytopenia (platelet count < 150,000 platelets per microliter of blood), a decrease in platelet count by 50% or more, venous or arterial thrombosis, necrotic skin lesions at heparin injection sites, or acute systemic reactions (e.g. fever/chills, tachycardia, hypertension, dyspnea, cardiopulmonary arrest) occurring after intravenous heparin administration. Thrombosis is the primary cause of morbidity and mortality in HIT; therefore, treatment should be initiated as soon as HIT is suspected [1, 2]. However, thrombocytopenia in critically ill patients is often multifactorial and may be due to various inciting events, making it difficult to distinguish HIT from non-HIT thrombocytopenia, especially in patients having cardiac surgery. Common causes of thrombocytopenia that mimic the presentation of HIT include circuit-related effects causing platelet activation and aggregation, sepsis, medications, surgery, bleeding, hemodilution, intravascular devices, and blood transfusion. The 4Ts score is recommended by the American Society of Hematology (ASH) to differentiate patients with HIT from other causes of thrombocytopenia. This tool incorporates the severity of thrombocytopenia, time of onset after heparin exposure, thrombosis and other clinical findings, and other potential etiologies to establish the pretest probability of diagnosis. With an intermediate or high probability, a presumptive diagnosis of HIT should be made, and treatment should be initiated before confirmatory testing. In the case of the patient we present, who experienced a platelet count fall of > 50% and nadir > 20k, clear onset of HIT between five and ten days after heparin exposure, new thrombosis, and no other apparent causes of thrombocytopenia, a 4Ts score of 8 indicated a high probability of HIT. Typical treatment for HIT includes stopping all heparin products, holding or reversing warfarin and starting a non-heparin anticoagulant [3].
Confirmatory testing for anti-PF4-heparin antibodies, also referred to as HIT antibodies, includes immunoassays (e.g., ELISA) and functional assays. Immunoassays detect the presence of these antibodies, reported in optical density (OD) units, but do not assess the ability of these antibodies to bind and activate platelets as functional assays do. Functional assays, such as the serotonin release assay and the heparin-induced platelet activation assay, are more specific than immunoassays and are therefore considered the gold standard for the diagnosis of HIT. The diagnosis is confirmed with a positive ELISA with an OD ≥ 2.00 (or ≥ 1.50 for patients with a high probability 4Ts score) or if there is a positive functional assay [4]. The patient in the current case presented positive for HIT anti-PF4/heparin IgG with an OD of 2.69, as well as a positive serotonin release assay.
Thrombocytopenia in HIT occurs due to the removal of IgG-coated platelets by hepatic and splenic macrophages [5]. Approximately 17% of patients treated with unfractionated heparin (UFH) and 8% treated with low-molecular-weight heparin develop anti-heparin-PF4 antibodies that me-diate HIT. Despite these statistics, only 20% of patients who test positive for these antibodies will develop thrombocytopenia, and even fewer experience platelet activation and thrombosis consistent with HIT (3% and less than 0.1%, respectively) [6].
Clinical manifestations of HIT are varied and may have significant implications for the affected patient. Thrombosis-related complications are most common, but bleeding may also occur more rarely. Deep vein thrombosis (DVT) and pulmonary emboli are among the more common complications seen in HIT among patients who are prone to venous thromboembolism [7]. In patients who experience HIT in the context of recent surgery, venous thrombosis is most common. Notably, in patients with comorbid cardiovascular disease, arterial thrombotic occlusion occurs at a greater rate. Such manifestations of HIT can result in de-leterious clinical outcomes, including skin necrosis, limb occlusion leading to amputation, and thrombosis of native or prosthetic valves [8]. More unusual sites of thrombosis can lead to outcomes such as mesenteric ischemia, cerebral infarction, visceral infarction, and myocardial infarction [9]. Though arterial occlusion is already at the rarer end of the spectrum of clinical manifestations of HIT, central arterial complications are rarer still than peripheral arterial complications such as limb ischemia [10]. Specifically, thrombosis of a native coronary artery is a severe and rare situation following HIT [11]. In the current case, the patient suffered 100% occlusion of the right coronary artery, a severe manifestation of an already rare sequela of HIT not often described in the relevant literature.
Extracorporeal life support is a the-rapeutic modality allowing temporary support in pulmonary and/or cardiac failure refractory to conventional clinical treatment. Extracorporeal membrane oxygenation (ECMO) is a primary extracorporeal life support measure used today. Venoarterial ECMO (VA-ECMO) may be utilized as a last-ditch effort in cases that require cardiac support, regardless of lung function, and typically serves as a bridge to recovery, left ventricular assist device, or transplantation. While the Extracorporeal Life Support Organization (ELSO) defines no absolute contraindication to the use of ECMO, appropriate patient selection is critical, and the risks and benefits should be considered individually. One of the main challenges in ECMO management is establishing a balance between hemostasis and thrombosis. The ELSO anticoagulation guidelines recommend that hemostasis should be evaluated before initiation in patients who are candidates for ECMO support. Intravenous unfractionated heparin is the gold standard of anticoagulation therapy for patients on ECMO due to its low cost and availability, easy titration, and reversibility with protamine. It is recommended that the heparin infusion be initially guided by the activated coagulation time (ACT) and subsequently titrated and guided by the activated partial thromboplastin time (aPTT) or anti-Xa activity [12]. When HIT is suspected, alternative methods of anticoagulation must be implemented.
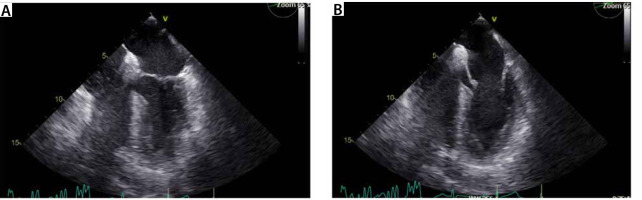
A 74-year-old male patient with a history of coronary artery disease status after stent placement in 2005, prostate cancer with PSA (prostate specific antigen) progression on leuprolide, obstructive sleep apnea on CPAP (continuous positive airway pressure), type 2 diabetes mellitus, hyperlipidemia, gout, and hypothyroidism presented to the emergency department with chest pain suggestive of unstable angina. He had a positive stress test and was scheduled for left heart catheterization and coronary angiography, which de-monstrated severe stenosis of the proximal left anterior descending artery, 80% stenosis of the 1st diagonal branch and diffuse moderate disease. The patient underwent uncomplicated two-vessel coronary artery bypass grafting (CABG) – left internal mammary artery to left anterior descending artery, saphenous venous graft to posterior descending artery (SVG to PDA) – and was referred to the intensive care unit (ICU) for recovery. The postoperative transesophageal echocardiogram (TEE) obtained before leaving the ope-rating room (OR) showed normal left ventricular systolic function without wall motion or valvular abnormalities (Figure 1).
The patient was on a heparin drip for 5 days pre-operatively, received 30,000 units of heparin intraoperatively and was continued on subcutaneous heparin for DVT prophylaxis post-operatively. He received 5,000 units of subcutaneous unfractionated heparin every eight hours for DVT prophylaxis. On postoperative day (POD) 2, he had an acute decrease in his platelet count to 137,000 from 243,000 the day prior. His platelets continued to decrease; on POD 6, the platelet count was 43,000 per microliter of blood. Subcutaneous heparin was suspended, standard postoperative dual anti-platelet therapy with aspirin and clopidogrel was held, and the patient was started on a bivalirudin infusion for an aPTT goal of 70–90 seconds. HIT anti-PF4/heparin IgG antibody panel returned positive with an OD of 2.69 (standard value < 0.4) and a positive serotonin release assay. On POD 6, the patient suffered an episode of atrial fibrillation with a rapid ventricular response and was started on an amiodarone infusion and resolved. Venous duplex on POD 9 demonstrated a superficial thrombus of his right basilic vein and a non-obstructive subacute DVT of his left common femoral vein.
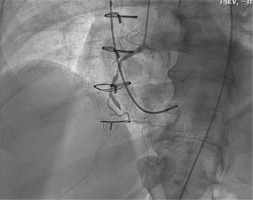
Transthoracic echocardiogram (TTE) on POD 9 showed a mildly reduced left ventricle (LV) ejection fraction compared to admission. The patient complained of nonspecific chest pain on ambulation with physical therapy, and an electrocardiography was obtained, which showed premature atrial contractions and 2 : 1 heart block with no new ST segment changes. Troponin peaked at 12 ng mL–1, creatinine remained stable, and platelet count was 39,000. He was brought to the cardiac catheterization laboratory to evaluate for acute bypass graft failure secondary to graft occlusion in the setting of HIT. Just before arterial access, the patient developed a complete heart block and became hypotensive, requiring support with dopamine and norepinephrine, and the decision was made to access the right internal jugular vein for temporary pacemaker wire placement under fluoroscopy. The patient became increasingly hypo-xic, so the anaesthesia team was called for assistance, and the patient’s trachea was intubated. Right femoral access was gained for the left heart cathe-terization, which demonstrated an occluded right coronary artery (previously stented in 2005) and complete occlusion of the SVG-PDA graft (Figure 2). Shortly after the patient went into pulseless electrical activity arrest, cardiopulmonary resuscitation was initiated, and return of spontaneous circulation was achieved after 16 minutes. The patient was periphe-rally cannulated via the right femoral artery and vein for initiation on VA-ECMO. The drainage site was the right femoral vein. He was then taken to the OR with the cardiothoracic surgery team in a stable condition.
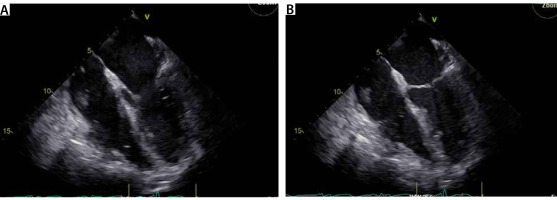
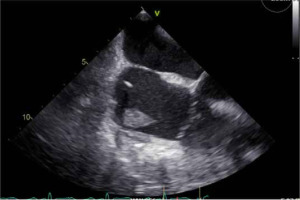
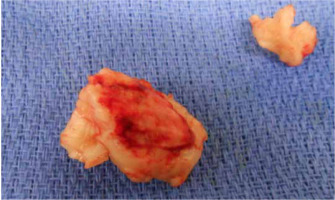
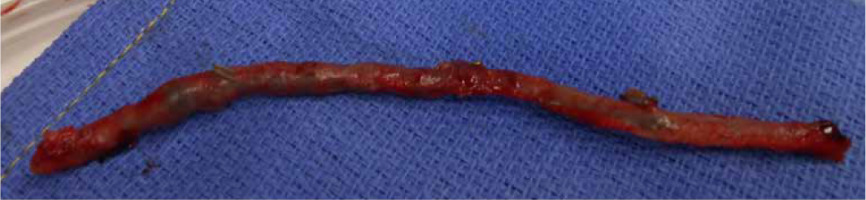
In the OR, TEE demonstrated mo-derate to severe mitral regurgitation, severe hypokinesis of the right ventricle (RV) and LVEF of 25% (Figure 3). There was a large thrombus in the right at-rium (Figure 4), patent foramen ovale with left to right flow, and an occluded saphenous venous graft to distal right coronary artery graft. A large white clot was removed from the right atrium (Figure 5) with no extension of the thrombus or residual thrombus in the left atrium. The atrial septum was closed with 4-0 prolene in double layers. The saphenous venous graft to the distal right coronary artery graft was carefully excised and inspected (Figure 6), showing the good backflow of blood from the anastomotic site. A white thrombus was found at the proximal and distal end, with some fresh clots in between. The patient underwent emergency CABG of the occluded SVG-PDA graft. The new anastomosis was a saphenous venous graft to the right coronary artery’s posterolateral branch (PLB), as the PLB was a better target than the posterior descending artery. Epicardial leads and an intra-aortic balloon pump (IABP) were placed before leaving the OR. The patient was transferred back to the ICU and remained intubated with an open chest. He stayed on VA-ECMO with a blood flow of 4.5 L min–1 and a sweep gas flow of 1.25 L min–1.
The patient was continued on a bivalirudin drip for anticoagulation management in the HIT setting. The patient did not exhibit severe renal dysfunction, though he did experience stage 1 acute kidney injury due to intravascular volume depletion secondary to blood loss from his surgery. Dialysis was not required, and bivalirudin treatment was not affected. The platelet count nadir was 7,000 per microliter of blood, and the patient received two rounds of plasma exchange with a 50 : 50 ratio of fresh frozen plasma and albumin on POD 10 and 12. On POD 14, the patient returned to the OR for ECMO decannulation, mediastinal washout and chest closure. TEE at that time showed improved LV function and improved but persistent RV dysfunction as well as moderate to severe mitral regurgitation. The patient was transferred back to the ICU, still requiring support with IABP, epinephrine, norepinephrine and vasopressin infusions. IABP was removed on POD 16.
On POD 17, TTE showed improved cardiac function with LVEF 45–50%, mild RV dysfunction with septal dyskinesis, mild mitral and tricuspid regurgitation, and estimated right ventricular systolic pressure in the mid-20s mmHg. The patient’s platelet count gradually recovered while anticoagulation was continued on a bivalirudin drip. Notably, the patient had not had an encouraging neurologic examination since ECMO cannulation. He had not followed commands or moved spontaneously since that time despite pauses in sedation. Neurology was consulted on POD 21, and an electroencephalogram was obtained, which showed moderate bihemispheric dysfunction, indicating abnormal activity in both cerebral hemispheres. The brain magnetic resonance imaging examination showed subacute ischemia involving the left precentral gyrus. With these findings and predicted slow recovery, the patient underwent tracheostomy and percutaneous endoscopic gastrostomy tube placement. Bivalirudin was discontinued on POD 28, and the patient was started on apixaban 5 mg twice per day. Trach collar trials began on POD 37, and the patient was discharged to a long-term rehabilitation facility on POD 51 due to severe deconditioning. The patient recovered remarkably well after discharge from the rehabilitation facility and was followed closely by an outpatient cardiology and anticoagulation clinic. Apixaban was discontinued after six months of therapy. TTE nine months after discharge demonstrated LVEF 60% and mild left atrial enlargement.
The case described presents a no-te-worthy and uncommon clinical scenario in which a patient developed HIT, resulting in severe thrombotic manifestations at atypical sites, eventually leading to clinical deterioration and cannulation for ECMO. Vascular thrombosis in patients with HIT most often involves the lower pressure venous circulation, presenting manifestations such as DVT and pulmonary embolism [8]. Venous thrombotic manifestations were seen in the current case, as the patient experienced a superficial thrombus of his right basilic vein and a non-obstructive subacute deep vein thrombosis of his left common femoral vein. Complications involving arterial circulation occur more rarely, with those involving central arterial circulation, as seen in the current case, being rarer still than those involving peripheral arterial circulation. Occlusion of central arterial circulation due to HIT may manifest in manners including cerebral infarction or myocardial infarction [10, 13]. Such a manifestation is seen in the current case, where the patient experienced post-CABG chest pain in addition to troponin levels which peaked at 12 ng mL–1 secondary to total occlusion of both his SVG-PDA graft and his native right coronary artery. There is a dearth of literature on cases like this, where HIT in a post-operative cardiac surgery patient leads to 100% occlusion of a native coronary artery and graft. The location and severity of thrombosis in this patient present a unique perspective on the identification and management of a rare manifestation of HIT.
In treating acute coronary syndromes in patients with HIT, strategies may include percutaneous intervention as well as thrombus aspiration, depending on the timing, location, and severity of thrombosis [14]. In terms of anticoagulation choice, no randomized controlled trials have compared non-heparin anticoagulants in patients with HIT and acute coronary syndromes. Small studies have shown efficacy in the use of bivalirudin and argatroban [15, 16]. Bivalirudin, the anticoagulation treatment used in the present case, has also been shown to be associated with a lower risk of bleeding [17]. In treating acute coronary syndromes in the context of HIT, the recommended regimen for bivalirudin is a bolus of 0.75 mg kg–1 followed by an intravenous infusion of 1.75 mg kg–1 hour–1. For argatroban, a bolus of 350 μg kg–1 should be followed by an intravenous infusion of 25 μg kg–1 min–1 titrated to ensure an ACT range of 300 and 450 seconds [18].
The risk of thrombosis in HIT is high enough that therapeutic-dose anticoagulation is needed regardless of whether a thrombotic event has occurred. A potential exception is an individual who has clinically significant bleeding or for whom the risk of bleeding is determined to be very high. Significant thrombocytopenia alone is not a contraindication to full-dose anticoagulation. Non-heparin anticoagulants that can be used in a patient with HIT include parenteral direct thrombin inhibitors (e.g., arga-troban, bivalirudin), danaparoid (not available in the United States), fondaparinux, or direct oral anticoagulants (DOACs) such as apixaban, rivaroxaban, edoxaban, or dabigatran [19, 20].
The choice of agent may be influenced by drug factors (availability, cost, ability to monitor the anticoagulant effect, route of administration, and half-life), patient factors (kidney function, liver function, bleeding risk, and clinical stability), and the experience of the clinician. In patients with critical illness, increased bleeding risk or increased potential need for urgent procedures, argatroban or bivalirudin may be preferred because of the shorter duration of effect. If delaying cardiovascular surgery or percutaneous coronary intervention (PCI) is not feasible, the ASH guideline panel suggests one of the following: intraoperative anticoagulation with bivalirudin, intraoperative heparin after treatment with preoperative and/or intraoperative plasma exchange, or intraoperative heparin in combination with a potent antiplatelet agent (e.g., prostacyclin analogue or tirofiban) [3].
In consideration of oral therapy, there is evidence supporting the use of DOACs rather than warfarin for oral anticoagulation in HIT. If a DOAC is used, it does not require platelet recovery to 150,000 as warfarin does. Evidence from observational studies suggests that DOACs may be effective in reducing thrombosis risk in HIT without stimulating HIT antibodies to bind to platelets or promote platelet aggregation. These agents may be a good option for individuals with HIT, either in the acute setting or for prolonged therapy. In some cases, the DOAC was given as initial therapy, while in others it was preceded by a parenteral agent such as argatroban or bivalirudin. Dosing of DOACs in HIT has not been well established based on clinical trials; therefore, dosing in this case was based on mechanistic understanding and placed a high value on avoiding thromboembolism. Since apixaban was started after a period of parenteral anticoagulation greater than seven days with bivalirudin, the recommended dose approved for initial venous thromboembolism therapy was 5 mg twice daily. Non-heparin anticoagulation should be continued for at least 4 weeks in patients without thrombosis and for at least 3 months in patients with thrombosis [3, 21]. The patient in this case was continued on apixaban 5 mg twice daily for 6 months after being transitioned off bivalirudin.
A rare exception to lifelong heparin avoidance may be an individual who requires a very brief course of heparin in the case of cardiovascular surgery. In patients with confirmed HIT antibodies who require cardiovascular surgery, there are several recommendations. If possible, delay surgery as HIT antibodies are present for approximately 100 days but may persist for longer [22]. Performing plasmapheresis prior to heparinization can remove heparin/PF4 antibodies, or alternatively, high-dose intravenous immune globulin may be administered preoperatively to reduce the activity of heparin/PF4 antibodies [23, 24]. Another potential option is to co-administer an anti-platelet agent with heparin immediately prior to or during the procedure. Such anti-platelet agents include tiro-fiban (glycoprotein IIb/IIIa receptor antagonist), cangrelor (P2Y12 inhibitor), or epoprostenol, with discontinuation of the infusion at the end of cardiopulmonary bypass or at the time of protamine administration to prevent platelet activation by the PF4/heparin antibodies [25–27]. Since intravenous epoprostenol causes vasodilation, a vasopressor infusion is typically necessary to maintain adequate systemic blood pressure [28]. Surgical procedures may also be performed using a non-heparin anticoagulant such as bivalirudin or argatroban. Both drugs are direct thrombin inhibitors with relatively short half-lives, approximately 25 minutes for bivalirudin, which is enzymatically and renally excreted, whereas argatroban is hepatically excreted [29–31]. Therefore, either option would have been an appropriate choice given that the patient had normal renal and hepatic function pre-operatively. Although the safety of bivalirudin has been well elucidated, the standardized management of bivalirudin administration in ECMO patients with HIT has not been clearly described.
The incidence of HIT in patients on ECMO is not well characterized. One study concluded that the prevalence of HIT among patients on VA-ECMO is extremely low at 0.36%, with an associated mortality rate of 33.3%, which is like patients on VA-ECMO without a diagnosis of HIT [32]. While most patients develop HIT after being on ECMO, the patient in this study already had a confirmed HIT diagnosis at the time of cannulation. Patients on ECMO who develop HIT have an even higher risk of developing thromboembolism than patients with HIT alone. As such, recognition of HIT in patients on ECMO is critical as it requires an urgent change in therapy. Many modern ECMO circuitry components contain heparin that is covalently bonded to albumin in the coating to reduce the immune response to circuit components. Therefore, if platelet recovery does not occur after the withdrawal of heparin, ongoing exposure from the heparin bonding in the circuit may be a factor, and one should consider removing any catheters or circuit components with heparin bonding if possible [33]. The 2012 American College of Chest Physicians HIT guidelines do not provide guidance for the diagnosis and management of HIT in patients on ECMO. However, they do recommend bivalirudin for patients with acute HIT who require urgent cardiac surgery over other non-heparin anticoagulants and heparin plus antiplatelet agents (grade 2C) [34]. It has been reported that there is no risk of HIT from bivalirudin or other direct thrombin inhibitors. Patients undergoing ECMO with HIT can improve platelet counts rapidly when switching to bivalirudin anticoagulation. Meanwhile, the pooled data showed that the incidences of bleeding events in patients with bivalirudin anticoagulation are comparable to those of UFH [35].
If heparin-dependent platelet anti-bodies are not present and the patient exhibits significant bleeding risk, ECMO can be facilitated without systemic anticoagulation via subcutaneous thromboprophylaxis or fon-daparinux [36, 37]. While current data and controlled trials comparing bivalirudin with other non-heparin anticoagulants during ECMO are limited, two studies (NCT03707418 and NCT03965208) investigating the efficacy and safety of bivalirudin compared to UFH in adult patients on ECMO are ongoing, and the results may shed more light on the topic [35]. Further research in this area and the development of standardized protocols for diagnosing and managing HIT in patients on ECMO support are warranted.