Intraoperative hypotension after induction or initiation of anaesthesia is a common complication in clinical practice. It has been associated with poor patient outcomes including increased peri-operative morbidity and even mortality [1]. Some special patient populations, such as the elderly and pregnant women undergoing caesarean section, are particularly prone to developing significant anaesthesia-induced hypotension (AIH) due to their unique physiological characteristics. Hypotension after induction of general anaesthesia (PIH, post- induction hypotension) or administration of spinal anaesthesia (PSH, post-spinal hypotension) is common and profound in patients with intravascular volume depletion [2]. It may result in nausea, vomiting, aspiration, dizziness, syncope, acute kidney injury and even cardiac dysrhythmias [3].
Preoperative intravascular volume optimization may help avoid hypotension after induction of anaesthesia. On the other hand, excessive intravascular volume loading might result in side effects of volume overload such as pulmonary oedema, congestive cardiac failure, renal dysfunction, and poor surgical outcomes. Hence, it would be of great value for anaesthesiologists to have a reliable tool for assessing intra-vascular volume status that can accurately predict the occurrence of AIH, so that preoperative fluid administration and optimization can be instituted for only those who will benefit from it.
There is no consensus yet on the optimal method for the assessment of preoperative intravascular volume status and fluid responsiveness [4]. Various non-invasive modalities such as transthoracic echocardiography (TTE), transthoracic bioimpedance, and the passive leg raising test (PLRT) have been investigated as tools for detecting intravascular volume depletion [4, 5]. Ultrasound (US) imaging of the inferior vena cava (IVC) is being used as a tool for assessing cardiac preload or intravascular volume status and has gained popularity because of its relative ease of use, feasibility, reproducibility, non-invasive nature, and cost-effectiveness. Respiratory phasic variation of the IVC diameter measured as a percentage decrease of IVC diameter at inspiration taking the expiratory diameter as baseline in a spontaneously breathing patient is known as the IVC collapsibility index (IVCCI). For long, it has been considered a reliable predictor of volume status and fluid responsiveness in critically ill patients until very recently a review questioned its utility [6]. Although several recent studies have assessed the role of IVCUS in predicting either PSH or PIH, there is no systematic review on this topic that summates the evidence. Whether IVCUS can be used to predict intraoperative hypotension remains an unanswered question. Therefore, the objective of this systematic review is to assess the role of IVCUS- derived parameters to predict PSH or PIH.
METHODS
This systematic review was conducted as per PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, and the protocol for this review was prospectively regi-stered [7]. Institute ethics committee approval was waived and informed consent from patients was not applicable due to the nature of the article.
Search strategy
Using a pre-defined search strategy #11 “((Inferior vena cava collapsibility index) OR (Inferior vena cava ultrasound) OR (IVC collapsibility index) OR (IVCCI) OR (Inferior vena cava diameter) OR (IVC variability) OR (IVC distensibility) OR (IVC collapsibility) OR (IVC spontaneous breathing) AND (Hypotension))” a literature search was conducted on the search engine PubMed from inception to 18.06.2022 (3:00 PM), along with a meticulous manual search.
Inclusion criteria
Observational studies and randomized control trials assessing the role of pre-operative IVCUS- derived parameters and PSH or PIH in adult patients were included in the review.
Exclusion criteria
Studies involving paediatric patients, those not published in the English language, and studies of which full texts were unavailable or in pre-proof stage were excluded from the review. No communication was made with the authors for the full text if it was not available.
Study selection
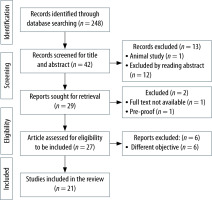
Initial screening of title and abstracts of the search results was followed by full-text screening of selected articles by SRC, PKD, and DR. In case of any disagreement regarding the inclusion of an article, the opinion of SM or DKB was sought. The study selection process for this review is depicted in Figure 1. Turconi et al. [8] investigated 55 high risk cardiac patients undergoing vascular surgery under GA and found IVCCI as a poor predictor of intraoperative hypotension. Aissaoui et al. [9] also found IVCCI > 42% as a poor predictor of hypotension after GA induction in 64 patients aged more than fifty years undergoing elective abdominal surgery. These two studies were excluded from our review as the full text of these studies were not available, but wherever applicable we have presented the data available from the abstract of these two studies.
Data extraction
Relevant data extraction was performed by SRC, SN and DR with respect to the study population, sample size, incidence of PSH or PIH, type of surgery, IVCUS technique, definition of PSH or PIH, drugs used for spinal anaesthesia (SA) or induction of general anaesthesia (GA), IVCCI cut-offs to predict PSH or PIH with sensitivity and specificity, additional parameters derived by IVCUS using the PICO (population, intervention, control, and outcomes) framework.
Methodological quality of study
Risk of bias and applicability concerns summary
Risk of bias at the study level was assessed as per QUADAS 2 methodology and it had four key domains: patient selection; index test; reference standard; flow and timing [10].
Effect measures
Primary outcome: Role of pre-operative IVCCI to predict PSH or PIH.
Secondary outcome: Role of other pre-operative IVCUS derived parameters to predict PSH or PIH.
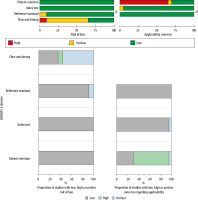
Risk of bias of individual studies was independently assessed by two review authors (SM & DKB) and reported in Figures 2 and 3 (green – low risk, yellow – unclear risk, red – high risk).
FIGURE 2
Risk of bias and applicability concerns summary: review authors’ judgements about each domain for each included study (green – low risk, yellow – unclear risk, red – high risk)

FIGURE 3
Risk of bias and applicability concerns graph: review authors’ judgements about each domain presented as percentages across included studies
Due to extreme heterogeneity of data, sensitivity analysis or meta-analysis was not done.
RESULTS
Study characteristics
Patient population and type of surgery
The role of IVCUS to predict PSH was assessed in 15 studies [11–25]. Among them 4 prospective observational studies evaluated PSH in a parturient undergoing caesarean section [11–14]. The age of the parturient varied between 18 and 40 years.
In studies among non-parturients, 4 studies were randomized controlled trials [15–17, 25] and the rest were observational studies [18–24]. These studies were conducted in procedures such as hernia and hydrocele [16], orthopaedic lower limb surgery [15, 20–22], and various other operations performed under spinal anaesthesia. These studies included adult patients between 18 and 75 years old [19]. Two studies recruited only elderly patients [22, 23]. Among the studies done in non-parturient patients, 8 studies excluded American Society of Anaesthesiology (ASA) class ≥ III [15–21, 24], whereas 1 study each included ASA class I–III [25], ASA class II–III [22]and ASA class I–IV [23] patients.
Six observational studies evaluated the role of preoperative IVCUS for PIH in non-pregnant patients undergoing elective surgery under GA [26–31]. All these studies were done in adult patients. The ASA class of included patients was I–II in three studies [27, 28, 31], I–III in one study [29], II–III in one study [30], while one study did not mention the ASA class of the patients [26].
Drugs used
The local anaesthetic drugs used for SA were both plain and hyperbaric bupivacaine, isobaric ropivacaine and levobupivacaine [11–25]. The most common adjuvant drug for SA was fentanyl. In one study 60 mg of buprenorphine was used [15]. For GA, 5 studies used propofol [26–28, 30, 31]. One study used etomidate [29]. Fentanyl was co-admini-stered at induction in all but one study [26].
IVC ultrasound technique
Low frequency curvilinear [11, 12, 14, 15, 19, 21, 23, 24, 29] or phased array [13, 22, 29] US transducers with the frequency range of 1–8 MHz were used for IVCUS in all these studies. IVC diameter was obtained at a point ranging between 0.5 cm [27] and 5 cm [12] from the IVC-right atrium (RA) junction. In some studies, the IVC diameters were obtained at points ranging between just proximal to [22], to 2 cm distal [20, 21, 30] to the junction of the hepatic vein and IVC.
Definition of hypotension
The definition of PSH varied widely among different studies: fall in SBP > 20% [11, 13], fall in SBP > 25% [15, 16, 25], fall in SBP > 30% [20], absolute fall in SBP > 50 mmHg [25], SBP value < 90 mmHg [24], SBP < 80 mmHg [25], fall in MAP > 20% [12, 14, 18], fall in MAP > 30% [17, 23], MAP < 65 mmHg [12, 14, 22], MAP < 60 mmHg [15–17, 24, 25], and fall in BP (unspecified) > 30% [19, 21]. The timeframe for PSH was defined as within 15 minutes after spinal drug administration in 3 studies [12, 18, 20], within 30 minutes in 2 studies [19, 23], within 45 minutes in one study [21], and until the end of surgery in two studies [11, 22]. The rest of the studies did not clearly define any timeframe in the definition of PSH.
The definition of PIH also varied widely: SBP < 90 mmHg or use of vasopressors within 21 minutes of propofol administration [26], MAP < 65 mmHg or fall in MAP > 30% [30], MAP < 65 mmHg or fall in MAP > 30% within 10 minutes of propofol administration [28], MAP < 60 mmHg or use of vasopressors until 15 minutes after GA induction [31], SBP < 90 mmHg or fall in SBP ≥ 30% [27], and fall in MAP > 30% or MAP < 60 mmHg until 10 minutes of tracheal intubation [29].
Incidence of hypotension
Incidence of PSH varied from 42.8% [13] to 76% [11] in pregnant patients undergoing caesarean section [11, 13] and from 18.3% to 45% in non- pregnant patients [17, 18, 21]. Incidence of post- induction hypotension varied from 19.3% in the study by Mohammed et al. [28] to 70% in the study by Fiza et al. [30].
Predictability of hypotension
IVCCI as a predictor of PSH had the highest sensitivity of 84.6% and the highest specificity of 93.1% in the same study (at a cut-off > 33%) [14]. The lowest sensitivity reported was 58.8% at a cut-off > 21.15% and the lowest specificity was 23.5% at a cut-off > 20.4% [12, 18].
IVCCI as predictor of PIH had the highest sensitivity of 86.67% and the highest specificity of 94.29% in the same study at a cut-off > 43% [31]. The lowest reported sensitivity was 45.5% and the lowest reported specificity was 77.27% both at a cut-off > 50% [26, 27].
Among the parameters other than IVCCI which were obtained from IVCUS, the IVC diameters and IVC peak velocity could not reliably predict PSH in 6 studies [11–13, 18, 20, 21]. However, one study found the minimum IVC diameter to be significantly lower in patients who developed subsequent hypotension [14]. In the study by Aslan et al. [23] the expiratory IVC diameter < 1.8 cm could predict PSH with an odds of 3.289. In another study, dIVCmax- to-IVCCI ratio, where dIVCmax was the maximum dia-meter of IVC at expiration, was a reliable predictor of PSH in elderly patients aged more than 70 years [22]. The maximum and minimum IVC dia-meters could not predict PIH in one study [28], but in another study the maximum IVC diameter of < 1.8 cm could predict PIH with moderate sensitivity (73.8%) and specificity (70.8%) [29]. The population charac-teristics, different definitions of anaesthesia- induced hypotension and the drugs used in different studies are presented in Table 1. Different me-thods of IVCUS and the important findings of the studies are presented in Table 2. The data collected from the abstract of the two studies which were excluded due to non-availability of the full text are also included in Table 1 and Table 2, as both these studies had the objective of our interest.
TABLE 1
Population characteristics, definition of anaesthesia-induced hypotension and drugs used in included studies
| S. no | Author and year | Study type, sample size | Population | Definition of intraoperative hypotension | Incidence of hypotension | Drugs used and dosage | ||
|---|---|---|---|---|---|---|---|---|
| 1 | Spinal anaesthesia | Pregnant patients | Yao et al., 2021 [13] | Observational 56 | Age 18–40 years parturient undergoing caesarean section | Fall in SBP > 20% from the baseline | 42.8% | 0.75% isobaric ropivacaine (12 mg) with fentanyl (10 mg) in spinal; 27-G Whitacre needle |
| 2 | Kundra et al., 2014 [11] | Observational 32 | Age 20–30 years parturient undergoing caesarean section | Fall in SBP > 20% by the end of surgery | 76.0% | 9 mg (0.5%) bupivacaine in spinal | ||
| 3 | Singh et al., 2019 [12] | Observational 40 | Parturient undergoing caesarean section | 20% fall in MAP or MAP < 65 mmHg within 15 minutes | 57.5% | 8 mg (0.5%) heavy bupivacaine with 20 mg fentanyl in spinal; 25G Sprotte needle | ||
| 4 | Elbadry et al., 2021 [14] | Observational 55 | 25–35 years parturient undergoing caesarean section | Fall in MAP > 20% from baseline or MAP < 65 mmHg | 47.3% | 12.5 mg heavy bupivacaine with 20 mg fentanyl; 25G spinal needle | ||
| 5 | Non-pregnant patients | Devi et al., 2021 [15] | RCT 100 | 18–60 years old, ASA class I–II patients undergoing orthopaedic lower limb surgery; one group received IVCUS guided fluid therapy (CI) and the other group did not undergo IVCUS (NCI) | 25% fall from baseline SBP or MAP < 60 mm Hg | 28.0% overall; 18.0% in CI group and 38.0% in NCI group | 12–15 mg heavy bupivacaine with 60 mg (0.2 mL) buprenorphine in spinal; 25G Quincke’s needle | |
| 6 | Ayyanagouda et al., 2020 [16] | RCT 80 | 18–60 years, ASA class I–II patients undergoing inguinal hernia and hydrocele surgery. Randomised to two groups – IVCUS guided fluid therapy (group A) and no IVCUS (group B) | 25% fall in baseline SBP or MAP < 60 mm Hg | 35.0% in Group A, 20.0% in group B | 15–18 mg 0.5% heavy bupivacaine; 25G Quincke’s needle | ||
| 7 | Ceruti et al., 2018 [25] | RCT 160 | 18–65 year, ASA class I–III patients non-cardiac, non- obstetric surgery under spinal anaesthesia; one group received IVCUS guided fluid therapy (group IVCUS) and the other group did not undergo IVCUS (group C) | Fall in SBP > 50 mmHg or 25% of baseline, absolute SBP < 80 mmHg, MAP < 60mmHg, 30% fall in MAP for more than 30 seconds | 35.0% overall, 27.5% in IVCUS and 42.5% in other group | 12–18 mg (0.5%) heavy bupivacaine with 27G pencil point spinal needle | ||
| 8 | Ni et al., 2022 [17] | RCT 90 | 18–65 years, ASA class I–II patients undergoing non-cardiovascular, non-obstetric surgery under spinal anaesthesia, Randomized to two groups – IVCCUS guided fluid therapy (IVCCI group) and standard fluid therapy without IVCCUS (Standard group) | Fall in MAP > 20% within 15 minutes after spinal anaesthesia | 23.8%; 31.7% in standard group and 15.3% in IVCCUS group | 12–15 mg 0.5% plain bupivacaine; 25G Quincke needle | ||
| 9 | Chowdhury et al., 2022 [18] | Observational 50 | Age 18–65 years, ASA class I–II patients undergoing elective infra-umbilical surgery | 30% fall from baseline BP (not clearly mentioned SBP/DBP/MAP) till 30 minutes | 34.0% | 12.5 mg 0.5% heavy bupivacaine with 25 mg fentanyl in spinal | ||
| 10 | Roy et al., 2021 [19] | Observational 129 | Age 18–75 years, ASA I–II patients undergoing elective surgery under spinal anaesthesia | Fall in SBP > 30% from baseline measured until 15 minutes after spinal anaesthesia | 19.4% | 12.5–15 mg 0.5% heavy bupivacaine in spinal; 25 or 27G Quincke’s needle | ||
| 11 | Spinal anaesthesia | Jaremko et al., 2019 [20] | Observational 60 | Age > 18 years , ASA class I–II patients undergoing knee joint replacement surgery | Fall in SBP more than or equal to 25% or MAP < 65 mmHg for 30 seconds until the end of surgery | 18.3% | 15–17 mg levobupivacaine in spinal | |
| 12 | Saranteas et al., 2019 [22] | Observational 70 | Elderly patients > 70 years; ASA class II–III, undergoing lower limb surgery under spinal anaesthesia | Fall in MAP > 30% from baseline within 30 minutes after spinal anaesthesia | 41.0% | 12–18 mg 0.75% plain ropivacaine in spinal; 22 or 25 G pencil point spinal needle; additionally to facilitate lateral positioning for spinal anaesthesia US guided PNBs were performed; 10–20 mg propofol administered before PNBs | ||
| 13 | Aslan et al., 2021 [23] | Observational 73 | Age > 65 years, ASA I–IV patients undergoing surgery under spinal anaesthesia | Fall in BP > 30% from baseline (SBP/DBP/MAP not mentioned) measured for 45 minutes | 38.4% | 12.5 mg 0.5% bupivacaine; 25G Quincke needle | ||
| 14 | Mačiulienė et al., 2018 [21] | Observational 60 | Age ≥ 18 years, ASA class I-II patients undergoing knee joint replacement surgery | Fall in MAP > 30% of the baseline value or any MAP < 60 mmHg; severe hypotension defined as MAP < 55 mm Hg | 18.3% | 15–17 mg levobupivacaine in spinal; 27-G spinal pencil type needle | ||
| 15 | Salama et al., 2019 [24] | Observational 100 | Age > 18 years, ASA class I–II patients undergoing elective surgery under spinal anaesthesia | SBP < 90 mmHg or fall in SBP > 30% of the baseline value or any MAP < 60 mmHg | 45.0% | 12 to 15 mg 0.5% hyperbaric bupivacaine in spinal; 26-gauge Whitacre needle | ||
| 16 | General anaesthesia | Turconi et al., 2022 [8] (only abstract available)** | Observational 55 | High risk cardiac patients undergoing vascular surgery under general anaesthesia | Three definitions of intraoperative hypotension were used: systolic blood pressure (SBP) less than 100 mmHg, mean arterial pressure (MAP) less than 60 mmHg, and a decrease in MAP greater than or equal to 30% compared to baseline | NA | NA | |
| 17 | Aissaoui et al., 2022 [9] (pre-proof, only abstract available)** | Observational 64 | Age > 50 years patients undergoing elective abdominal surgery under GA | SBP < 90 mmHg or MAP < 65 mmHg or fall in MAP > 30% from pre-induction level | 51.0% | NA | ||
| 18 | Au et al., 2016 [26] | Observational 40 | Age > 18 years, patients undergoing elective surgical procedures under general anaesthesia | SBP < 90 mm Hg or use of vasopressors within 21 minutes after administration of propofol | 45.0% | Propofol dose at the discretion of the anaesthesiologist; mean dose: 2.4 mg kg–1; no mention of fentanyl or MR | ||
| 19 | General anaesthesia | Fiza et al., 2020 [30] | Observational 63 | Adult patients, ASA class II–III undergoing non-cardiac surgery under GA | MAP < 65 mmHg or fall in MAP > 30% from preoperative value | 70.0% according to definition by MAP < 65 mmHg, 68.0% according to fall in MAP, 49.0% for both definitions | Mean (SD) propofol dose 1.86 mg kg–1 (0.34 mg kg–1) with mean fentanyl 125 mg at general anaesthesia induction; 93.7% patients received fentanyl; 73% patients received midazolam 1–2 mg before induction; no mention of MR | |
| 20 | Purushothaman et al., 2020 [31] | Observational 50 | Age > 18 years, ASA classes I and II patients undergoing surgery under general anaesthesia with propofol as induction agent | MAP <60 mmHg or use of rescue vasopressors till 15 minutes after GA induction | 30.0% | Midazolam 0.05–0.1 mg kg–1, propofol 1–2 mg kg–1 with fentanyl 2–3 mg kg–1, atracurium as muscle relaxant 0.5 mg kg–1 | ||
| 21 | Mohammed et al., 2021 [28] | Observational 110 | Age 18 to 50 years, ASA class I and II patients undergoing surgery under GA with propofol for induction | MAP < 65 mmHg or fall in MAP > 30% from baseline to 10 minutes from GA induction; significant hypotension MAP < 55 mm Hg or fall in BP > 40% or hypotension duration ≥ 2 min | 19.3% | Midazolam, fentanyl, propofol, MRs (not specified) dose at the discretion of anaesthesiologist | ||
| 22 | Szabo et al., 2019 [27] | Observational 83 | Age > 18 years, ASA class I–II patients undergoing elective general surgery under general anaesthesia with propofol induction | SBP < 90 mmHg or ≥ 30% drop of SBP from the baseline | 34.1% in patients with IVCCI > 50% and 24.2% in patients with IVCCI < 50% | 1.5–2 mg kg–1 propofol with 1-2 mg kg–1 fentanyl and rocuronium or cis-atracurium | ||
| 23 | Zhang et al., 2016 [29] | Observational 90 | Adult patients, ASA class I to III patients undergoing elective surgery under general anaesthesia with etomidate for induction | Fall in MAP > 30% from baseline or MAP less than 60 mmHg until 10 minutes after tracheal intubation | 46.7% | 0.01 mg kg–1 midazolam, 0.3 mg kg–1 etomidate with 2–3 mg kg–1 fentanyl with cis-atracurium or rocuronium | ||
TABLE 2
Method of IVC US and important findings of included studies
| S. no | Author and year | Method of IVCUS | Parameters derived by IVCUS | Diagnostic accuracy of IVCCI | AUROC IVCCI | Diagnostic accuracy of other parameters derived from IVC US | ||
|---|---|---|---|---|---|---|---|---|
| 1 | Spinal anaesthesia | Pregnant patients | Yao et al., 2021 [13] | Cardiac probe (5–1 MHz); subxiphoid view 2–3 cm from right atrium, anteroposterior diameter and peak velocity of IVC (supine position), IVCCI not seen; measurements taken at end-expiration | AP diameter (maximum and minimum) and peak velocity of IVC | – | NA | AP diameter and peak velocity of IVC failed to predict PSH |
| 2 | Kundra et al., 2014 [11] | 3–5-MHz curvilinear probe, checked in 3 different positions – supine, wedged recumbent and left lateral; measurements taken at end-expiration and end-inspiration at all positions | IVC diameter (maximum and minimum) and IVCCI | IVCCI > 13.6% in recumbent with wedge position predicts PSH with a positive predictive value of 86% | NA | End expiratory IVC diameter was not a reliable predictor of PSH | ||
| 3 | Singh et al., 2019 [12] | Curvilinear 3.5–5 MHz probe, subcostal view, supine position with and without wedge, 3–5 cm distal to RA, just proximal to IVC and hepatic vein junction; measurements taken at end-expiration and end-inspiration at both positions | IVC diameter (maximum and minimum) and IVCCI | IVCCI > 25.64% with 60.9% sensitivity and 35.5% specificity for to predict PSH for supine with wedge position; IVCCI > 20.4% with 69.6% sensitivity and 23.5% specificity for non-wedged position | 0.46 (wedged position); 0.38 (without wedge) | Maximum and minimum IVC diameter could not predict PSH | ||
| 4 | Elbadry et al., 2021 [14] | Curvilinear USG probe (1–5 MHz, 21 mm), 3–4 cm away from RA just distal to the hepatic vein; after expiration and inspiration | IVC diameter (maximum and minimum) and IVCCI | IVCCI > 33% can predict PSH with 84.6% sensitivity and 93.1% specificity | 0.95 | Minimum IVC diameter significantly lower in hypotensive group | ||
| 5 | Non-pregnant patients | Devi et al., 2021 [15] | Curvilinear probe of 2–5 MHz, subcostal view, 2–3 cm from RA; measurements taken at maximum end-expiration and at end-inspiration | IVCCI | No correlation between IVCCI cut-off 40% and PSH | NA | – | |
| 6 | Ayyanagouda et al., 2020 [16] | Curvilinear probe 1–8 MHz, subcostal view, 2 cm from RA; measurements taken in spontaneously breathing patients | IVCCI | – | NA | No correlation between IVCCI > 36% and baseline MAP | ||
| 7 | Ceruti et al., 2018 [25] | 3S-RS probe, 4 MHz, subcostal view 4 cm from right atrium; measurements taken in spontaneously breathing patients at inspiration and expiration | IVCCI | Slight correlation (r2 = –0.16) between IVCCI > 36% and reduction of MAP after spinal anaesthesia | NA | – | ||
| 8 | Chowdhury et al., 2022 [18] | 2–5 MHz probe, supine position, 1cm distal to the joining of hepatic vein and IVC; measurements taken during breathing without deep inhalation at 12-15 breaths per minute | IVC diameters (maximum and minimum), IVCmax/IVCCI | IVCCI > 21.15% predicts PSH with 58.8% sensitivity, 69.7% specificity | 0.6 | No correlation between maximum and minimum IVC diameter and PSH; IVCmax/IVCCI > 60 predicted PSH with 58.8% sensitivity and 54.5% specificity | ||
| 9 | Spinal anaesthesia | Roy et al., 2021 [19] | Curvilinear probe 3.5-5 MHz, subxiphoid view, 2-3 cm distal to IVC-RA junction; measurements taken both at expiration and inspiration | IVCCI | Non-significant correlation between IVCCI and PSH (r2 = 0.025) | 0.467 | – | |
| 10 | Jaremko et al., 2019 [20] | Probe not mentioned; 1–2 cm below the level of hepatic veins; supine position with one leg bent; measurements taken at breathing rate 16(± 2) [mean(± SD)] | IVC diameters (maximum and minimum) and IVCCI | IVCCI could not predict PSH | < 0.7 | Expiratory and inspiratory IVC diameters failed to predict PSH | ||
| 11 | Saranteas et al., 2019 [22] | 2–5 MHz phased array probe, IVC diameters measured just proximal to the entry of hepatic veins; dIVCmax measured at end-expiration but IVCCI measured at spontaneous quite breathing | IVCCI and dIVCmax/IVCCI | IVCCI > 30% predicts PSH with 61% sensitivity and 82% specificity | 0.77 | dIVCmax/IVCCI < 43 predicts PSH with 80.5% sensitivity and 93% specificity | ||
| 12 | Aslan et al., 2021 [23] | Curvilinear probe 2-5MHz, subxiphoid view; supine position | IVC diameter(maximum and minimum) and IVCCI | – | 0.77 (for end-expiratory diameter) | Expiratory IVC diameter < 1.8 cm can predict PSH with odds of 3.289 | ||
| 13 | Mačiulienė et al., 2018 [20] | Curvilinear 2-6 MHz, subcostal view, 1–2 cm below the level of the hepatic vein, supine position with one leg bent; hypotension and IVCCI were measured at base-line (before SA) and at 4 subsequent time points: 1. Immediately after SA; 2. 15 minutes after SA; 3. 30 minutes after SA; 4. 45 minutes after SA; measurements taken at normal breathing at 14 ± 2 min-1; [mean(± SD)] | IVC diameter(maximum and minimum) and IVCCI | IVCCI > 50%, does not predict PSH | IVCCI as predictor of hypotension at: 1. Time point-1: AUROC 0.59 2. Time point-2: AUROC 0.57 3. Time point-3: AUROC 0.44 5. Time point-4: AUROC 0.51 | Expiratory and inspiratory IVC diameters and IVCCI failed to predict PSH | ||
| 14 | Ni et al., 2022 [17] | Subcostal view, 2 to 3 cm distal to RA; measurements taken at the end of expiration and inspiration during same respiratory cycle | IVC diameter (maximum and minimum) and IVCCI | IVCCI > 42% can predict PSH w ith 83.9% sensitivity and 76.3% specificity | 0.834 (IVCCI cut-off > 42%) | Patients with smaller IVC diameter developed PSH more frequently | ||
| 15 | Salama et al., 2019 [24] | Curvilinear 3.5 to 5 MHz probe, subxiphoid view, 3 to 4 cm distal to RA just distal to IVC-hepatic vein junction; measurements taken at the end of expiration and inspiration during same respiratory cycle | IVC diameters (maximum and minimum) and IVCCI | IVCCI > 44.7% can predict PSH with sensitivity of 84%, a specificity of 77% and accuracy of 84% | 0.86 | – | ||
| 16 | Turconi et al., 2022 [8] (only abstract available)** | NA | IVCCI poor predictor of intra-operative hypotension | 0.62 | ||||
| 17 | Aissaoui et al., 2022 [9] (pre-proof, only abstract available)** | NA | IVCCI > 42% poor predictor of post-induction hypotension | 0.68 | ||||
| 18 | General anaesthesia | Au et al., 2016 [26] | Subxiphoid or intercostal window; 2 cm below entry of hepatic veins into IVC; to avoid off-midline measurements M-mode employed in the transverse plane; measurements taken over one normal respiratory cycle | IVC diameter (maximum and minimum) and IVCCI | IVCCI > 50% predicts post induction hypotension with 66.67% sensitivity and 77.27% specificity | NA | IVCCI ≥ 50% had odds ratio of 6.9 in predicting post induction hypotension | |
| 19 | Fiza et al., 2020 [30] | Subcostal view, 2 cm caudal to the hepatic vein-IVC junction; measurements taken at end-expiration and end-inspiration | IVC diameter (maximum and minimum) and IVCCI | Higher IVCCI was associated with increased incidence of PIH for each definition separately with odds of 1.05 for each definition; with both definitions of hypotension taken simultaneously, the IVCCI was not a predictor of hypotension | NA | – | ||
| 20 | Purushothaman et al., 2020 [31] | Subxiphoid view, 2–3 cm distal to RA; measurements taken in spontaneously breathing patients | IVC diameter (maximum and minimum) and IVCCI | IVCCI of > 43% good predictor of post-induction hypotension with 86.67% sensitivity and 94.29% specificity | 0.959 | IVCCI > 50% had 53.33% sensitivity and specificity of 100% | ||
| 21 | Mohammed et al., 2021 [28] | 2.5–5 MHz phased array probe, subcostal view, 2–3 cm from RA; readings were taken during a single spontaneous breathing cycle | IVC diameter (maximum and minimum) and IVCCI | IVCCI ≥ 46% had 47 to 59% sensitivity and 48 to 50% specificity for hypotension; 0.46 (significant hypotension) | 0.51 (post induction hypotension) dIVCmin cut-off 0.73 sensitivity 47% to 53% and specificity 50% | dIVCmax ≥ 1.42 cm sensitivity 47% to 53% and specificity 51 to 53% | ||
| 22 | Szabo et al., 2019 [27] | 5 MHz probe, subxiphoid view, 0.5–3 cm from the RA; if suboptimal view, trans-hepatic lateral view/intercostal view were taken; measurements taken during normal breathing in lightly sedated patients | IVC diameter (maximum and minimum) and IVCCI | IVCCI > 50% had poor sensitivity of 45.5% but high specificity of 90.0% for PIH | 0.648 | – | ||
| 23 | Zhang et al., 2016 [29] | Curved linear phased array probe, subcostal view, 2 to 3 cm distal to RA; medium sweep speed kept; measurements taken over single breathing cycle | IVCCI; IVCmax | IVCCI cut-off 43%, had sensitivity of 78.6% and a specificity of 91.7% for predicting post induction hypotension; IVCCI 38 to 43% considered as grey zone | 0.9 grey zone for dIVCmax 1.5 to 2.1 cm | dIVCmax cut off 1.8 cm had a sensitivity of 73.8% and specificity of 70.8% for predicting PIH | ||
Discussion
In this systematic review, we have come across contrasting results from studies using IVCUS for prediction of AIH. Heterogeneity with respect to the patient populations under evaluation, definitions used for hypotension after anaesthesia, IVCUS assessment methods, and cut-off values for IVCUS-derived parameters to predict hypotension precluded pooled meta-analysis. The maximum and minimum reported sensitivity of the IVCCI for predicting PSH was 84.6% and 58.8% respectively, while the maximum and minimum specificities were 93.1% and 23.5% respectively. For the prediction of hypotension after general anaesthesia (GA) induction, the reported ranges of sensitivity and specificity of IVCCI were 86.67% to 45.5% and 94.29% to 77.27%, respectively.
The cause of this wide disparity in results is multi-factorial. Firstly, there is a lack of consensus on the definition of hypotension under anaesthesia. Klöhr et al. [32], in an elaborate review of available literature on hypotension under spinal anaesthesia during caesarean section, found at least 15 different definitions of ‘hypotension’ in the 63 publications retrieved. This resulted in a wide range of the incidence of PSH from 7.4% to 74.1%. The authors reported that even minor changes of the definition cause major differences in the observed incidence of hypotension. This makes comparison between studies difficult and pooling of results nearly impossible.
The present review found 11 different definitions for PSH and 6 for PIH. The timeframe for defining PSH also varied widely, from within 15 minutes of the spinal injection to until the end of surgery. During operations under SA, the most vulnerable period for hypotension is the initial few minutes following sub-arachnoid injection of local anaesthetic. Hypotension occurs primarily due to loss of sympathetic tone in the lower half of the body leading to decreased systemic vascular resistance (SVR) due to arteriolar vasodilation, and loss of venous tone leading to reduced mean systemic filling pressure (MSFP) and reduced venous return [33]. As baroreceptor reflex mechanisms gradually restore SVR and blood volume gradually redistributes, venous return is restored, and blood pressure slowly returns toward baseline. Intravenous fluid boluses and short acting vasopressors are usually used to cover this period of sudden hypotension. There is, however, no specific definition for this period in the literature. After 15–30 minutes of spinal injection, the surgical procedure would obviously have started. At this stage, surgical causes such as positioning, retraction and blood loss will confound blood pressure changes. Therefore, until what time PSH can be attributed to sympatholysis by the SA is a matter of conjecture. Similarly, there is hardly any consensus on the definition of PIH in terms of magnitude or timeframe, in patients undergoing GA. Another important aspect of defining PSH or PIH is what should be considered the baseline value. It is very often observed that even normotensive patients, when wheeled into the operating room, have high blood pressure readings, probably because of preoperative stress or anxiety. Whether such blood pressure readings immediately prior to anaesthesia initiation should be considered as baseline or whether the “usual” blood pressure readings obtained during the pre-anaesthesia visit are to be considered remains unclear.
Aside from differences in definition of hypotension, there was remarkable heterogeneity in different aspects of the studies. The study populations in various studies were diverse. IVC dimensions and indices may be different between parturient and non-parturient patients. The IVCUS imaging and measuring techniques used in various studies were different. While a few studies have used a phased-array probe, others have used the curvilinear probe. The exact point at which the IVC diameter was measured in various studies was inconsistent. None of the studies have specified the M-mode sweep speed used during IVC diameter measurement. This may have affected the number of respiratory cycles sampled for measuring minimum and maximum IVC diameter.
The change in IVC diameter with phases of respiration depends not only on intra-vascular volume but also on the depth of respiration [34, 35]. The deeper the inspiratory effort is, the greater is the fall in intrathoracic pressure and consequent collapse of the IVC. Multiple factors including anxiety, pain, and metabolic acidosis may influence the respiratory efforts in an awake patient and can cause significant variations in the IVC diameter. It is difficult to standardize or quantify the respiratory efforts in a spontaneously breathing patient. Out of the 23 studies screened in this review, 5 have mentioned that measurement was performed during normal quiet breathing. Others however do not specify the breathing pattern during IVCUS. Studies that have reported a correlation between the attenuation of IVC collapsibility and elevated central venous pressure recommend that patients be asked to take a sudden sharp inspiratory effort, also described as a “sniff” test, during inspiratory IVC diameter measurement [36,37]. It is hypothesized that the sniff manoeuvre can standardize the inspiratory effort and, due to its brief nature, can be performed effectively with ease by both normal and dyspnoeic patients. Whether such a manoeuvre was performed in any of the studies in the present review is not clear.
IVC diameter and phasic variability with respiration have been shown to increase with age [38]. Thus, age too can be a confounding factor when comparing studies on IVC indices.
The heterogeneity in the above discussed factors was probably responsible for the conflicting results reported in the studies in this review. Consequently, a very wide range of individual cut-offs, sensitivities and specificities have been reported, making pooled analysis impossible. Hence, we have not proceeded further with a meta-analysis of the data.
In a meta-analysis of studies predicting fluid responsiveness in critically ill patients using the caval index, Orso et al. [6] reported a pooled sensitivity and specificity of 0.71 and 0.75 respectively. However, they pooled data from various studies using different cut-offs, and therefore did not recommend a clear cut-off for the caval index that can accurately predict fluid responsiveness.
The major limitation in our systematic review is that we could not perform a meta-analysis as we did not find enough studies taking the same cut-off values of IVCCI. That has precluded any comparison between age groups or between the pregnant and non-pregnant population, to find out whether IVCUS is useful in a specific subgroup of patients.
In conclusion, the results of studies assessing the utility of IVCCI for predicting AIH are very inconsistent with respect to both the ideal cut-off and dia-gnostic accuracy. IVCCI may be a simple, safe, and useful tool for predicting PSH and PIH. However, as IVC diameter and collapsibility are dependent on the interplay of multiple additional physiological factors such as intra-thoracic pressure, intra-abdominal pressure and venous compliance, a fixed cut-off may not always be reflective of the intravascular volume status. Hence, IVCUS derived parameters should be used in the appropriate clinical context to predict AIH. High-quality studies are needed with standardized methodology in terms of age group, patient population, IVCUS techniques and definition of hypotension before clear recommendations can be made.