Septic shock is a leading cause of mortality in critically ill patients, despite the use of new antibiotics and resuscitation therapies [1, 2]. Given the significance of timely beginning of appropriate antibiotics to optimise septic shock outcomes [1, 2], clinicians must quickly achieve a diagnosis of infection. In fact, it is now accepted that starting an early effective antibiotic therapy in the course of an infection decreases morbidity and mortality in this specific condition [2, 3]. Moreover, apart from diagnosing sepsis, clinicians are also faced with the mission of monitoring the patients’ responses to therapy toward either the resolution of the illness or its progression into a multisystem organ failure and finally death.
For all of these reasons, there is a need to identify biomarkers of sepsis in order to help clinical evaluation, differentiate between bacterial and non-bacterial infection, and to differentiate those patients who are most likely to recover from those who face a rather bad outcome. So far, many biomarkers have the potential to play a decisive role by providing adjunctive information to guide clinicians to a rapid diagnosis and to adjust a treatment. There are hundreds of biomarkers that could be potentially used for diagnosis and prognosis in septic patients [3–5]. To the best our knowledge, procalcitonin (PCT) and C-reactive protein (CRP) have been the most widely investigated [1, 2]. Moreover, it was well established that in severe sepsis and/or septic shock, there is a stimulation of the parasympathetic system, mainly the cholinergic anti-inflammatory pathway, to inhibit the release of the tumour necrosis factor (TNF) and to control the overwhelming inflammation [2, 4, 5]. Recently, it was established that low serum cholinesterase (SChE) activity is correlated with the diagnosis of septic shock due to bacterial infections [2, 4, 5]. Moreover, decreased SCHE activity is associated with mortality in critically ill patients [4, 5].
Moreover, in the intensive care unit (ICU), a serial measurement of sepsis biomarkers has been used as an adjunctive tool and as a guide to discontinue the antimicrobial therapies earlier [6–8]. However, to date, a few studies have evaluated the discriminative power of PCT, CRP, and SChE kinetics as predictors of survival outcome in patients admitted to the ICU with septic shock [2, 4, 5, 8–12]. In this study, we aim to explore the value of PCT, CRP, and serum SChE activity kinetics as useful predictors of mortality in patients with septic shock admitted to the ICU.
METHODS
We conducted a prospective study in the ICU of a university hospital over a period of 1 year (1st January to 31st December 2017). This study was approved by the institutional review board, and the requirement for written informed consent was waived by the ethics committee.
The study included all patients 18 years of age or older, with septic shock [13]. Septic shock was defined according to the Third International Consensus Definitions for Sepsis and Septic Shock [13]. All included patients were monitored with an arterial catheter and examined with echocardiography (by the same physician).
A documented infection was diagnosed when there was a clinically evident source of infection. The diagnosis of bacterial infection was confirmed when bacteria were isolated in various bacterial samples (blood samples, urine, sputum cultures, etc.). In our study, microbiological analyses were performed on blood samples, urine and sputum cultures, and specimens taken from other sites according to the clinical suspicion regarding the source of sepsis. All the patients underwent chest radiography and/or CT scanning as requested.
Patients were excluded when any of the following conditions were met: all patients admitted and transferred within 48 hours and during the weekend; patients with previous diagnosis of chronic and acute liver disease, cirrhosis, active tuberculosis, cancer, and malnutrition; organophosphate poisoning; brain herniation; and/or declared brain-dead. Moreover, patients with cardiogenic shock and those with hypovolemic shock (haemorrhagic shock) were excluded. Finally, we excluded all the patients with co-occurrence of cardiogenic and septic shock. Patients with viral and/or fungal infection were also excluded.
The clinical data were prospectively collected for each patient using pre-printed case report forms. For all included patients, a data entry form was designed to collect demographic, clinical, biological, and radiological data on admission and during their ICU stay. The biochemical parameters measured on admission and during the ICU stay were arterial blood gases and acid-base status (pH and bicarbonate), blood urea nitrogen (BUN), creatinine blood, leukocytes count, haemoglobin concentration, and coagulation profile. The severity of illness was assessed by simplified acute physiology score (SAPS II) calculated within 24 h of admission [14], and according to the SOFA (Sepsis-related Organ Failure Assessment) score calculated on ICU admission and during ICU hospitalization [15]. During the ICU stay, all complications were recorded: nosocomial infections [16], thrombocytopaenia, gastrointestinal bleeding, arrhythmias, and metabolic complications. Patients were followed until ICU discharge or death. All vital signs, standard laboratory variables, results for cultures of specimens from new infection sites, dose of vasopressors, and interventions were recorded daily.
Biomarkers
For all included patients, blood samples of septic biomarkers (PCT, SChE activity, and CRP) were obtained. Serum was collected for CRP, SChE activity, and PCT assays upon ICU admission (day 0: D0), at the day of septic shock (day 1: D1), then 3 and 5 days after the septic shock development (D3 and D5, respectively). SChE activity dosage was measured in not frozen serum, using a biochemistry analyser (Cobas 6000 analyser: module c501) with spectrophotometric method; the normal range values vary from 5320 to 12290 UI L-1. CRP was measured with immunoturbidimetric method and PCT with electrochemiluminescence method (ECLIA) using a Cobas 6000 analyser (e 601). In the current study, the laboratory teams were unaware of the patients’ evolution. All the biomarker assays were processed at the same laboratory.
Statistical analysis
Data were entered centrally by a healthcare worker using SPSS version 18.0 for Windows. Cate-gorical data were expressed in proportion and subgroups, while continuous variables were expressed as means ± SD. Risk factors were evaluated in univariate analysis and by multivariate analysis by a multiple logistic stepwise regression procedure.
Subgroups (survivals and deaths) were analysed using the χ2 test (or Fisher’s exact test), the 2-group t-test, or the Mann-Whitney U test, as appropriate. The level of significance was set at P < 0.05. We used multivariable logistic regression with a backward procedure. Non-redundant variables selected by univariate analysis (P < 0.05) and considered clinically relevant were entered into a logistic regression model. Odds ratios were estimated from the b coefficients obtained, with respective 95% confidence intervals (95% CI).
Sensitivity, specificity, and predictive values of SChE activity, PCT, and CRP for discrimination between survivals and deaths were calculated. Moreover, kinetics of SChE activity, PCT, and CRP were defined by the variation of values between the day of septic shock development (D1), D3, and D5. They were calculated by the ratio of the difference between the second and the first measurement (e.g. [value on D3 – value on D1]). These variables were then defined as Δ-CRPD1-D3 and Δ-CRPD1-D5, respectively.
The best cut-off values for serum SChE activity, PCT, CRP, Δ-CRP, Δ-PCT, and Δ- serum-ChE were chosen as the values that optimized sensitivity and specificity. Receiver operating characteristic (ROC) curves were plotted, and the respective areas under curves were calculated [17]. In a ROC curve, the true-positive rate (sensitivity) is plotted against the false-positive rate (1-specificity), with the area under the curve being proportional to the probability of a correct discrimination.
RESULTS
Descriptive characteristics and outcomes of patients
During the study period, 60 patients were included and 92 patients were excluded (Figure 1). The mean age was 47.7 ± 19 years. There were 46 male (74%) and 14 female (26%) patients. Mean SAPSII on ICU admission was 40.7 ± 16 (median: 37) and mean SOFA score on ICU admission was 16 ± 4 (median: 7). Moreover, 44 patients (73%) had one or more comorbidities. The most common past medical conditions were as follows: arterial hypertension in 20 patients (33%), diabetes mellitus in 18 (30%), chronic heart disease in 17 (28.3%), and chronic obstructive pulmonary disease (COPD) in 9 (15%). On ICU admission, 36 patients (60%) developed circulatory failure requiring a vasopressor administration (noradrenaline) to maintain a mean arterial pressure of 65 mm Hg or greater. Moreover, 57 patients (95%) had a respiratory failure requiring invasive mechanical ventilation on ICU admission. Finally, 26 patients (43.3%) developed acute kidney injury (AKI) on ICU admission (the moment they were admitted). During their ICU stay, all patients developed septic shock due to a bacterial infection. The most common sites of infection were the lungs in 36 patients (60%), followed by abdominal infections in 6 (10%), wound infections in 5 (8.3%), urinary tract infections in 4 (6.6%), bloodstream infections in 4 (6.6%), and other localizations in 5 cases (8.3%). The most commonly identified pathogens were as follows: Klebsiella pneumoniae in 12 patients (20%), Acinetobacter baumannii in 11 (18.3%), Pseudomonas aeruginosa in 6 (10%), and Escherichia coli in 4 (6.6%).
Within the study period, out of the 60 included patients, 37 died (61%). No patients died before the second sample was taken, and 55 patients survived until the 5th study day.
The comparison between the 2 groups (deaths and survivors) showed that the factors associated with poor outcome were age, higher SOFA score on ICU admission, and the need for invasive mechanical ventilation (Table 1).
TABLE 1
Factors correlated with death in univariate analysis
The value of sepsis biomarkers and their kinetics in the prediction of outcomes
The analysis of biomarkers of sepsis plasma concentrations showed that there was no difference in the mean of plasma SChE activity, nor in PCT and CRP plasma concentrations between survivors and non-survivors on ICU admission (D0) and the day of septic shock (D1) (Table 2). Moreover, as shown in Table 2, the comparison of mean plasma SChE activity, PCT, and CRP plasma concentrations (on D3 and D5) between survivors and non-survivors showed a significant decrease in mean PCT and CRP values between admission, D3, and D5 (P < 0.05) (Figures 2 and 3). Furthermore, a significant increase of mean SChE activity value between admission, D3, and D5 was found in survivors, compared with non-survivors, with a significant difference (P < 0.05) (Figure 4). In addition, a SChE activity value greater than > 3270 UI L-1 on D5 was associated with good outcome with sensitivity at 71%, specificity at 91%, and area under the curve (AUC) at 0.83.
TABLE 2
Comparison of sepsis-biomarkers between survivors and non-survivors
FIGURE 2
Evolution of C-reactive protein (CRP) levels in survivors (in black) and non-survivors (in grey). Results are expressed as mean, and upper and lower 95% CIs
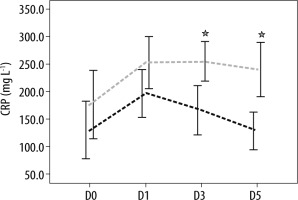
FIGURE 3
Evolution of procalcitonin (PCT) levels in survivors (in black) and non-survivors (in grey). Results are expressed as mean, and upper and lower 95% CIs

FIGURE 4
Evolution of serum cholinesterase (SChE) activity levels in survivors (in black) and non-survivors (in grey). Results are expressed as mean, and upper and lower 95% CIs

ROC analysis identified Δ-PCT (D1–D5) greater than 7.8 mg dL-1 (area under the curve: 0.65; S: 35% and Sp: 85%) and Δ-CRP (D1–D5) greater than 49 mg L-1 (AUC: 0.62; S: 57% and Sp: 75%) as the most accurate cut-offs in predicting a good outcome. On the other hand, we found that Δ-SChE activity (D1–D5) greater than 820 UI L-1 was associated with a bad outcome, with a sensitivity of 71%, a specificity of 81%, and an AUC at 0.83 (Figure 5). Moreover, a significant positive correlation was found between Δ-PCT (D1–D3) and Δ-SChE activity (D1–D3) (P = 0.001, R = –0.44), and between Δ-PCT (D1–D5) and Δ-SChE activity (D1–D5) (P = 0.026, R = –0.33) (Figure 6). The multivariate analysis showed that the factors associated with the poor outcome (death) were age over 63 years (P = 0.04; OR: 1.08; 95% CI: 1.004–1.16) and Δ-SChE activity (D1–D5) greater than 820 UI L-1 (P = 0.01; OR: 1.003; 95% CI: 1.0–1.005).
DISCUSSION
Sepsis and septic shock are among the leading causes of morbidity and mortality among critically ill patients; thus, the detection of prognostic factors is fundamental to determine their outcome.
Almost all included patients in our study showed elevated CRP and PCT and low SChE acti-vity levels on ICU admission. However, the initial measurements of these inflammatory biomarkers failed to distinguish between the survivors and the non-survivors. On the other hand, our study confirms that the kinetics of PCT, CRP, and SChE activity can be used as predictors of mortality in patients with septic shock admitted to the ICU. As a consequence, our study results highlight the validity and prognostic value of the kinetics of biomarkers of sepsis (PCT, CRP, and SChE activity in our study) in the detection of high-risk, life-threatening complications in patients who suffer from septic shock due to a bacterial infection. Moreover, a significant positive correlation was found between Δ-PCT and Δ-SChE activity on day 3 and day 5. As a consequence, the dosage of SChE activity can be used as an additional biomarker of sepsis to establish the positive diagnosis of septic shock due to bacterial infections, and their kinetics can be used as prognostic biological parameters.
In addition, we found in the current study that the deceased group was definitely older, and age was associated with poor outcome (death) in multivariate analysis. This can be explained by the high frequency of past medical conditions (arterial hypertension, diabetes mellitus, chronic heart disease, and COPD) in this subgroup.
The high mortality rate in our study can be explained by the severity of included patients on ICU admission and during ICU stay. In fact, Mean SAPSII on ICU admission was 40.7 ± 16 (median: 37) and mean SOFA score on ICU admission was 16 ± 4 (median: 7). Moreover, 57 patients (95%) had a respiratory failure requiring invasive mechanical ventilation and 26 patients (43.3%) developed acute kidney injury (AKI) on ICU admission (the moment they were admitted).
Sepsis is a heterogeneous syndrome characterized by a complex immune-inflammatory response to presumed or proven infection [18]. Early identification and intervention are crucial to improve sepsis outcomes. Leaving clinical diagnosis away, in many situations the laboratory diagnostics (sepsis biomarkers) may represent a helpful tool to predict the positive diagnosis of sepsis and/or septic shock [2, 3, 6]. There are hundreds of biomarkers that could be potentially used for diagnosis and prognosis in septic patients [3]. However, few studies have evaluated the discriminative power of PCT, CRP, and SChE activity kinetics as predictors of survival outcome in patients admitted to the ICU with septic shock [2–5, 10–12].
The prognostic value of CRP and PCT kinetics has been studied in several types of infection, with mortality as the main outcome variable, with contradictory results [8–11, 19, 20]. In our study, we found a significant decrease of mean PCT and CRP values on D3 and D5 in comparison with D1 (day of the septic shock) in survivors compared with non-survivors. In fact, when a patient improves under symptomatic treatment and antibiotics, the inflammatory reaction decreases, and the value of these sepsis biomarkers should decline.
More recently, it was established that the SChE activity level significantly decreases in patients with septic shock due to bacterial infections [2, 4, 5, 12, 21]. Moreover, the reduction in the SChE activity was detected significantly earlier compared to those of routinely measured inflammatory biomarkers [22]. In fact, the reduction in SChE activity was observed as early as 1–2 hours after the inflammatory and/or sepsis onset [22]. The pathophysiology of the rapid decline of SChE activity in patients with septic shock is not well-understood, and several hypotheses have been postulated [2]. In all cases, the role of inflammatory reaction is well-established [2, 4, 5, 12]. The first hypothesis is that during the progression of the sepsis, bacteria and their toxins activate neutrophils to release various substances (such as proteases, cytokines, etc.) affecting liver function with reduced synthesis of SChE. The second is hypothesis is that the dilution effect due to fluid therapy leads to a decrease in the plasmatic concentration of SChE. The third is the increased catabolism of SChE and the inhibition of SChE by inflammatory mediators (cytokines). Yet another hypothesis is that increased transcapillary loss of SChE is due to the increase of capillary permeability caused by sepsis. In a recent prospective study, Bahloul et al. [2] showed that decreased SChE activity can be used as a reliable diagnostic marker for septic shock. More recently, it has been established that the serum cholinesterase (SChE) activity level can be used as prognostic biomarker in septic shock [4, 5, 10]. In a prospective observational study [4], Zivkovic et al. [4] showed that the SChE enzyme activity was markedly reduced in all septic patients during the observation period, and the measured SChE enzyme activity in non-survivors was notably lower than that of the surviving patients for the duration of 28 days. Moreover, a continued SChE activity reduction, observed in non-surviving patients, symbolizes a novel warning indicator in the critical care setting, allowing simple and rapid detection of high-risk septic patients. Our study confirms the results of these studies [4, 5, 12]; in fact, we found a significant increase of mean SChE activity values between admission, D3, and D5 in survivors, compared with non-survivors. Moreover, we found that a decrease of Δ-SChE activity (D1–D5) by more than 820 UI L-1 was associated with a bad outcome with good sensitivity (71%) and specificity (81%).
When a patient improves under symptomatic treatment and antibiotics, the inflammatory reaction decreases, and SChE activity increases with time [2, 4, 5, 12]. However, in severe cases with multi-organ failure with worse outcome, the inflammatory reaction will be more pronounced, explaining the progressive decrease of SChE activity. However, to define the cut-off value, it is necessary to perform prospective studies, although interpreting SChE activity levels from diverse laboratory protocols remains a challenge. In our study we found a significant positive correlation between Δ-PCT and Δ-SChE; as a consequence, our study suggests the use of SChE activity as a new sepsis biomarker as an adjunctive tool.
The small size of our sample and the high rate of excluded patients can represent a methodological limitation. However, our study represents the second large study exploring the prognostic value of serum cholinesterase activity in septic shock, after the study published by Peng et al. [5]. Moreover, many patients died before the second sample could be taken; hence, our hypothesis could not be tested in all the patients.
CONCLUSIONS
Our study suggests that in a group of critically ill patients with severe septic shock, a rise or no change in procalcitonin and/or CRP level, and/or a decrease or no change in SChE activity should warn the clinician about the insufficiency and/or inadequacy of the therapy. However, a fall in procalcitonin and/or CRP levels, and/or a rise in SChE activity were associated with a favourable prognosis. Based on our study and some other data detailed above, we recommend that an estimation of SChE activity, procalcitonin, and CRP on the day of septic shock, followed by estimation within the next 72–120 h, could help the prognostic assessment of critically ill patients with septic shock. Further studies are needed to define the critical values related to mortality.







