Introduction
Among the most common cancers, lung cancer is a malignant disease with high morbidity and mortality. According to GLOBOCAN, there were 2.2 million new cases and 1.76 million deaths worldwide in 2020 [1]. Treatment of lung cancer is based on histopathology, molecular biological characteristics, disease stage, performance status (PS), and individual financial condition. Most lung cancers are non-small cell lung cancers (NSCLC). For this type of histopathology, when the disease is in the late stage, systemic treatments are preferred, to prolong the survival time and improve the quality of life of the patient [2]. In accordance with developments in molecular biology, many gene mutations have been identified that are associated with the pathogenesis of lung cancer, of which the most common are epidermal growth factor receptor (EGFR) mutations. Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKI), including erlotinib, are an effective first-line therapy for advanced EGFR-mutant NSCLC patients, with many advantages over chemotherapy [3–5].
According to epidemiological statistics in Vietnam, about 80% of newly diagnosed lung cancer cases are at stages III and IV, with distant metastases [6]. Brain metastasis is common in advanced NSCLC, occurring at the rate of approximately 25–40% during disease progression. NSCLC patients with brain metastases typically have poor prognosis, with a short median survival of 1–5 months if left untreated; they may receive symptomatic treatment with glucocorticoid therapy alone or whole-brain radiation therapy (WBRT). An important factor leading to the poor prognosis of lung cancer patients with brain metastases is the drug’s inability to cross the blood-brain barrier to reach the tumour [7–9].
Over the past 20 years, there have been many advances in the treatment of NSCLC. The identification of gene mutations that promote the proliferation of NSCLC cells, such as EGFR, ALK, ROS-1, RET, and MET, has led to the development of novel targeted therapies. It is especially important to mention that EGFR-TKIs have demonstrated superior effectiveness in EGFR-mutant patients, including higher response rates, longer survival time, and fewer adverse reactions, compared with traditional chemotherapy [3–5, 10–12]. These drugs have a small molecular weight, which leads to improved effectiveness when treating metastatic brain lesions, due to their ability to cross the blood-brain barrier. In addition, evidence has shown that EGFR-mutant NSCLC patients are more likely to develop brain metastases, at a rate of 44–63% [13, 14]. Therefore, the effectiveness and safety of EGFR-TKIs in EGFR-mutant NSCLC patients with brain metastases is a topic that should be explored and discussed.
We conducted this study to address the shortage of studies on the effectiveness of EGFR-TKIs with or without locoregional treatment in EGFR-mutant NSCLC patients with brain metastases.
Material and methods
This study was conducted in patients with stage IV EGFR-mutant NSCLC and brain metastases. The patients received 150 mg of erlotinib daily as first-line treatment at the Vietnam National Cancer Hospital from April 2019 to October 2022.
The research was conducted solely for the purpose of improving the effectiveness of treatment. The treatment regimen has been proven effective in many clinical trials around the world, and it has been approved by the FDA and is recommended in international and Vietnamese guidelines. All procedures involving participants were performed in accordance with the institutional and/or national research committee’s ethical standards, and personal information was kept confidential. The study protocol was reviewed and approved by the Hanoi Medical University Institutional Review Board (IRB-VN01001 approval no. NCS30/HMU-IRB). Informed consent was waived by the Ethics Committee of Hanoi Medical University due to the study’s retrospective nature and the fact that the analysis used anonymous clinical data.
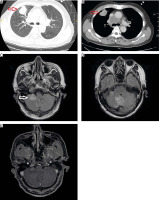
Treatment modalities included γ knife surgery (GKS), WBRT, and brain surgery. The treatments were approved by a multi-disciplinary team of oncologists, neurosurgeons, radiation oncologists, and radiologists. The evaluation criteria for treatment decisions included the number of lesions, the location and size of the metastatic brain lesions, age, PS, and other individual factors in most patients. Other combination therapies included treatment for bone metastases (e.g. radiotherapy to relieve pain and prevent compression, bone resorption inhibitors), radiotherapy to prevent mediastinal compression, and symptomatic treatment (i.e. analgesia, anti-cerebral oedema treatment, and anticonvulsants). The patients underwent clinical assessment during treatment. If symptoms improved or stabilized and undesirable effects were absent or acceptable, the patient continued treatment and follow-up. Every 2–3 months or following abnormal clinical events, computed tomography of the chest and abdomen were performed. Brain lesions were monitored through brain magnetic resonance imaging (MRI), and the duration of the MRI depended on the specific patient, as indicated by the doctors (Fig. 1).
Fig. 1
Representative computed tomography and magnetic resonance images of a non-small cell lung cancer patient: tumour was detected in the upper right lung (red arrow) (A, B), brain metastases (white arrow) (C, D, E)
Patient characteristics, including clinical and laboratory characteristics, age, gender, PS, histopathology, type of EGFR mutation, central nervous system (CNS) symptoms, number and size of metastatic brain lesions, combination locoregional brain therapy, survival time, and adverse reactions during treatment, were collected.
Statistical analysis
Data were collected through medical records, and the collected data were processed to produce medical statistics using SPSS 22.0 software (IBM Corp., Armonk, NY, USA). Quantitative variables are presented as mean and standard deviation. To compare the difference between 2 mean values, Student’s t-test was used if the distribution was normal, and the Mann-Whitney U test was used if the distribution was not normal. Qualitative variables are presented as percentages. To compare the differences between the 2 rates, the χ2 test was used if less than 20% of expected frequencies were ≤ 5 and there were no cells with expected frequency < 1; Fisher’s exact test was used if more than 20% of the expected frequencies were 5 or there were cells with frequency < 1. Survival time was analysed using the Kaplan-Meier method. The log-rank test was used to compare the difference in survival likelihood with relevant factors. To evaluate the independent factors affecting the dependent variables, multivariate Cox regression analysis was performed. The difference was statistically significant when p < 0.05.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding authors.
Ethics statement
This study was approved by the Ethics Committee of Hanoi Medical University (accession no. NCS30/HMU-IRB).
RESULTS
Patient population
In our study, 72 EGFR-mutant NSCLC patients with brain metastases received erlotinib 150 mg daily as first-line treatment. Table 1 summarizes the patients’ characteristics (clinical and laboratory). Of the 72 patients, 34 were female (47.2%), the mean age was 56.8 years, 60 patients (83.3%) had PS 0–1, 44 patients (61.1%) had never smoked, and 100% of the histopathology results were adenocarcinoma. Forty-one patients (56.9%) had 1-–3 brain lesions, and 31 patients (43.1%) had 4 or more brain lesions. Thirty patients (41.7%) had CNS symptoms. Epidermal growth factor receptor exon 19 deletion was present in more than 80.6% (n = 58) of patients. When the disease progressed, 34 patients (70.8%) developed the acquired mutation T790M and received the third-generation EGFR-TKI (osimertinib) as second-line treatment (Table 1).
Table 1
Patient characteristics
Twenty-two patients (30.5%) received EGFR-TKI monotherapy, and the other patients received EGFR-TKI plus locoregional brain treatment, including 32 patients who received GKS, 16 patients who received WBRT, and 2 patients who received both GKS and WBRT after brain surgery. In the EGFR-TKI monotherapy group, 14/22 patients had disease progression after being treated with erlotinib. 2/14 patients had T790M positive, then they were given osimertinib and closely monitored for brain lesions. The remaining 12 patients received chemotherapy, and 3 of them had gamma knife surgery. Patients who received EGFR-TKI monotherapy had significantly better Eastern Cooperative Oncology Group scale (ECOG) PS (p = 0.008), fewer CNS symptoms (90.9% vs. 9.1%, p = 0.0001), and fewer brain metastases (p = 0.0001) than those who received locoregional treatments (Table 1). Patients who received WBRT had more brain metastases than those who received GKS (Table 2).
Table 2
Characteristics of patients based on locoregional treatment modalities
Treatment results
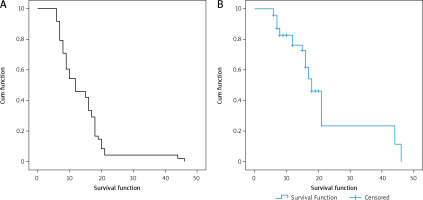
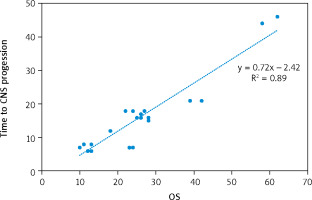
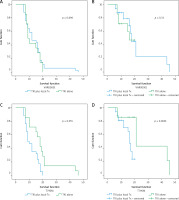
The median progression-free survival (PFS) and overall survival (OS) of the cohort were 11 months and 25 months, respectively (Fig. 2). The median time to CNS progression was 13.9 months in 48 patients, with progression determined by brain MRI. Of these 48 patients, 32 received GKS and 16 received WBRT. The median OS was significantly longer for patients who harboured the exon 19 deletion mutation than for those who had other mutations (26 months vs. 15 months, p = 0.023). There was no significant difference in the median OS or PFS between patients who received EGFR-TKI monotherapy and those who received EGFR-TKI plus locoregional treatments (26 vs. 24 months, p = 0.33; and 12 vs. 10 months, p = 0.696, respectively). Median PFS was significantly longer for patients who had ECOG PS 0 to1 compared with those who had ECOG PS of 2 (15 vs. 7 months, p = 0.039) (Table 3). Patients with T790M mutation after EGFR-TKI resistance had significantly longer median OS than those without the mutation (41 vs. 23 months, respectively, p = 0.0001) (Fig. 3). Other clinical and laboratory characteristics did not influence PFS or OS. There was no significant difference in PFS or OS between patients who received GKS and those who received WBRT. Patients with longer time to CNS progression had significantly longer median OS, R2 = 0.89 (Fig. 4).
Table 3
Progression-free survival and overall survival of all patients
Fig. 3
Progression-free survival (PFS) and overall survival (OS) of each group: PFS and OS of epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) with locoregional treatment for brain metastases vs. EGFR-TKI monotherapy (A, B), PFS and OS of patients with T790M (–) vs. T790M (+) (C, D)
Discussion
This study evaluated the treatment results of EGFR- mutant NSCLC patients with brain metastases for whom EGFR-TKI alone or in combination with locoregional treatment was indicated as the first-line therapy at Vietnam National Cancer Hospital, Hanoi, Vietnam. The median PFS, OS, and time to CNS progression in our study were in line with other studies. In addition, we found that the exon 19 deletion is a good prognostic factor for patients, resulting in longer survival time compared with other mutations [15–18].
Epidermal growth factor receptor tyrosine kinase inhibitor combined with locoregional treatment in EGFR-mutant NSCLC patients with brain metastases is controversial. Although many retrospective studies reported contradictory results [19–28], some meta-analyses suggest that combination therapy can improve OS [29, 30]. First, many studies have reported high radio sensitivity of EGFR-mutant NSCLC [27, 30]. Second, radiation can affect the blood-brain barrier, which leads to an increase in TKI permeability, contributing to the effectiveness of the treatment [19, 28–30].
In clinical practice, studies have shown that the EGFR-TKI plus GKS combination prolongs OS compared with the EGFR-TKI plus WBRT combination [29, 30]. Although patients who receive WBRT have larger and more numerous brain metastases and poorer PS, there is no difference regarding PFS or OS between them and patients who do not receive WBRT. These results suggest that WBRT may be an option for patients who are no longer indicated for stereotactic radiosurgery.
In contrast with previous studies that demonstrated the benefit of the combination of EGFR-TKI with brain locoregional brain treatment, there was no significant difference in PFS or OS between the 2 groups in our study. The results could be explained by the higher percentage of asymptomatic patients with brain metastasis BM in the EGFR-TKI alone group and the lack of a substantial difference in outcomes between the EGFR-TKI alone group and the local treatment group in patients who did not exhibit CNS symptoms. Other retrospective studies have investigated the advantages of combining locoregional brain therapy with EGFR-TKI; however, comparing these studies to the current one is challenging because some of them focused on patient cohorts treated with WBRT alone or included patients who had been on second- or third-generation EGFR-TKI, which influenced the treatment results [22, 24, 26, 27].
In our study, patients who progressed after receiving first-generation EGFR-TKI treatment because of the appearance of a T790M acquired resistance mutation had significantly better survival outcomes than patients with other mutations. The reason for this is that all these cases continued to be treated with osimertinib, a third-generation EGFR-TKI. This is consistent with studies around the world that reported superior effectiveness of third-generation EGFR-TKI compared with first- and second-generation EGFR-TKIs regarding clinical response rate, PFS, and OS; furthermore, the third-generation EGFR-TKI is especially effective in patients with brain metastases [12]. In the FLAURA trial, which compared osimertinib to gefitinib or erlotinib, in the subgroup of patients with CNS lesions at baseline the median CNS PFS (counting only CNS progression or death as events) was not reached for osimertinib and was 13.9 months for earlier generation EGFR – tyrosine kinase inhibitor. The objective response rate was 66% with osimertinib vs. 43% with gefitinib or erlotinib (odds ratio, 2.5; 95% CI: 1.2–5.2; p = 0.011). Therefore, first-line treatment with osimertinib is the best option for EGFR-mutant NSCLC patients according to the guidelines. Nowadays, in countries where third-generation TKI is available, the use of first-generation TKI in brain metastases should be discouraged due to concerns about its ability to penetrate brain parenchyma. However, in Vietnam, osimertinib is not reimbursed by the Health Insurance Fund; it remains unaffordable for most patients because of the high price, while first-generation EGFR-TKI has been reimbursed 50% by the Health Insurance Fund for over 10 years. Therefore, in actual clinical practice in our country, first-line treatment with a first-generation EGFR-TKI is a more affordable option and is more feasible for patients pursuing long-term treatment.
Our study is one of the first to evaluate the effectiveness of EGFR-TKI treatment with and without locoregional brain treatment for EGFR-mutant NSCLC patients with brain metastases in a large central facility with a sufficiently long follow-up. Many studies have been conducted to evaluate the effectiveness of EGFR-TKI; however, there was heterogeneity in their study populations, including in the use of first-, second-, and third-line treatments or multiple EGFR-TKIs. Therefore, the results of this study accurately reflect the treatment results in practice in Vietnam. Doctors can reference the findings of this study when choosing treatment options in clinical practice.
Our study has several limitations. First, it was a retrospective study that was conducted in only one facility, and the sample size was not sufficiently large. Second, due to the retrospective nature of the study, we were unable to investigate all the events during treatment, namely CNS symptoms, such as neurocognitive dysfunction, and their impact on quality of life. Finally, because brain scans are performed according to the doctor’s discretion, in many cases, the time to CNS progression could not be accurately assessed.