Dear Editor,
A 50-year old man, never smoker, non-allergic, suffering from chronic gastritis, dyslipidemia, and idiopathic pulmonary fibrosis (IPF), diagnosed two years ago, came to our attention with global chronic respiratory failure due to acute exacerbation of IPF with clinical conditions in decline characterized by increasing dyspnea, edema and drowsiness. A high-resolution computed tomography (CT) scan showed an accelerated IPF model (Figure 1).
FIGURE 1
Computed tomography scan shows definable distorted ground glass component with evident traction bronchiectasis in lower lobes, medium lobe and lingula, a marked progressive distortion and calcific bone metaplasia in distortion areas configuring an accelerated idiopathic pulmonary fibrosis pattern

The functional tests showed a severe reduction in the diffusion lung capacity of carbon monoxide (DLCO) equal to 26%. Laboratory tests show-ed neutrophil leukocytosis and high inflammatory markers (VES and acute-phase protein C [PCR]). We administered antibiotics, corticosteroids and diuretic therapy. On admission to hospital, systemic arterial blood oxygen gas with a flow of 9 L min-1 showed severe respiratory failure with hypercapnia: pH 7.32; pCO2 79 mm Hg (11 kPa); pO2 46 mm Hg (6 kPa). The patient refused non-invasive ventilation (NIV) and we administered the treatment of high-flow nasal cannula (HFNC) with a flow rate of 60 L min-1 and a fraction of inspired oxygen (FiO2) of 0.56. After four hours the level of pCO2 was reduced to 73 mm Hg (10 kPa) and pO2 increased to 55 mm Hg (7 kPa). So, we performed serial measurements of gas values in the following days and we obtained a gradual fall of hypercapnia. The monitoring of blood gas values in the following days of hospitalization showed an improvement of respiratory failure after 8 days equal to 66 mm Hg (9 kPa) pCO2 and pO2 of 60 mm Hg (8 kPa) with FiO2 of 0.33; after 12 days pCO2 was 58 mm Hg (8 kPa), pO2 59 mm Hg (8 kPa) and pH of 7.41 with FiO2 0.32. With the use of HFNC pH in arterial blood gases gradually returned to the physiological range. FiO2 was slowly reduced until it reached a value of 0.3, and stabilization of pCO2 of approximately 55 mm Hg (7 kPa) was found. The X-ray images of the chest showed a slight improvement during hospitalization (Figure 2) and laboratory tests showed a reduction in neutrophil leukocytosis and inflammatory markers.
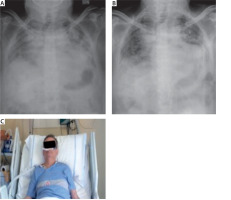
FIGURE 2
Chest X-ray. A) Chest X-ray at admission to hospital. B) Chest X-ray after the treatment, at the end of hospitalization. C) Image of patient in treatment with high-flow nasal cannula
The interest of our clinical case is essentially due to the use of a high-flow nasal cannula for the treatment of patients with acute exacerbation of idiopathic pulmonary fibrosis with higher levels of pCO2. Typically, in various studies analyzing the effect of HFNC respiratory failure CO2 values do not exceed 55 mm Hg (7 kPa). In 2000, idiopathic pulmonary fibrosis (IPF) was defined as a specific form of chronic interstitial pneumonia, progressive, fibrosis of unknown cause that occurs primarily in older adults, usually around fifty or sixty, and limited to the lungs [1–4]. The IPF incidence increases with age, with a presentation typically constituted by insidious onset of dyspnea [4]. Rarely, in patients with IPF acute exacerbation may present as the initial event [4]. Acute exacerbation (AE) of IPF is an acute, clinically significant respiratory deterioration characterized by evidence of newly developed widespread alveolar abnormality [5]. Acute exacerbations of IPF (AEx-IPF) occurs in approximately 5–10% of patients with diagnosed IPF annually [1]. One-year and three-year incidences of AEx-IPF are 14.2% and 20.7%, respectively [1]. AE is the most common cause of death in patients with IPF [5]. The diagnostic criteria for AE-IPF were the following: 1. Previous or concurrent diagnosis of IPF; 2. Acute worsening or development of dyspnea typically < 1 month duration; 3. CT scan showing new bilateral ground-glass opacity or consolidation superimposed on a background pattern consistent with usual interstitial pneumonia; 4. Pulmonary deterioration not fully explained by cardiac failure or fluid overload [2]. Several hypotheses for the etiology of AEx-IPF have been proposed [1]. AEx-IPF may represent a sudden acceleration of the underlying disease process due to unknown acute injury to the lung, or a biologically distinct pathological process due to a clinically occult condition, such as infection or gastroesophageal reflux disease (GERD) [1]. An AEx-IPF can occur at any time during the course of IPF [1]. Median survival after an AEx-IPF has been reported to be between 22 days and 4.2 months [1]. Reported in-hospital mortality rates vary widely, between 27% and 96% [1]. Patients with an AEx-IPF had a lower median survival time than those who had not suffered an AEx-IPF and lower 5-year survival rates (18.4% vs. 50.0%) [1]. There is some evidence that patients with better lung function (FVC, PaO2, DLCO) prior to AEx-IPF are more likely to survive an AEx-IPF, suggesting that preservation of lung function may be an important way of reducing the impact of AEx-IPF in patients with IPF [1]. Supportive care remains the mainstay of treatment for AEx-IPF, but also gives a weak recommendation for the treatment of the majority of patients with AEx-IPF with corticosteroids, based on anecdotal reports of benefit and the high mortality associated with AEx-IPF [1]. Corticosteroids are used in the majority of patients who suffer an AEx-IPF, usually in pulse doses [1]. Broad-spectrum antibiotics and immunosuppressants (cyclosporin or cyclophosphamide) are sometimes used in addition to corticosteroids [1]. However, the efficacy of immunosuppressants in the treatment of AEx-IPF is based on a few small retrospective studies that do not provide conclusive evidence for benefit [1]. Mechanical ventilation is often used in patients with AEx-IPF, but the data on its effects on outcomes are mixed [1]. AEx-IPF may lead to severe, refractory hypoxemia [2]. In a limited number of cases, significant CO2 retention, which is caused by the ‘stiffness’ of a fibrotic lung, may also occur [2]. High-flow nasal cannula (HFNC) oxygen therapy, which delivers heated, humidified inspired gas at a high flow rate of up to 60 L min-1 and a precise fraction of inspired oxygen (FiO2), is being increasingly used to correct severe, refractory hypoxemia in patients with respiratory distress due to a variety of causes. It has, in fact, been found to improve patients’ oxygenation, respiratory rate (RR), heart rate, and dyspnea score [2, 3]. In terms of respiratory physiology, HFNC is reported to have benefits such as a decrease in dead space by flushing out the expired gas accumulated in the anatomical dead space of the nasopharynx and muco-ciliary movement of the respiratory tract [3]. It has also been reported that intra-airway and alveolar pressures are increased by flowing oxygen at a high flow rate, resulting in a positive end-expiratory pressure (PEEP)-like effect [3]. The respiratory physiological effects of HFNC have been reported in regard to its effect in flushing out dead spaces, decreasing nasal and pharyngeal mucosal resistance, the PEEP effect (1–3 cm H<sub>2</sub>O [0.1–0.3 kPa]), increasing alveolar ventilation, improving warming and humidification, and improving the reliability of the inspired oxygen concentration [3]. HFNC use appears to reduce the work of breathing [3]. It was indeed reported that the work of breathing is reduced during HFNC use as thoracoabdominal synchrony becomes better than with a face mask [3]. Accordingly, it is thought that HFNC use is not merely an oxygen therapy, and it exerts a spontaneous ventilatory assistance effect in terms of the respiratory physiology [3]. The only study that has assessed the effect of these new technologies on the outcomes of patients with AE-IPF is a case series showing that HFNC is well tolerated, leads to higher ventilation efficiency and lower RR, and reduces the work of breathing [2]. There are several factors that could explain why the treatment algorithm proved effective in improving the short-term prognosis of our patients with AE-IPF. First, HFNC appears to have played a role in reversing hypoxemia [2]. Considering that severe hypoxemia caused by a marked ventilation/perfusion (V/Q) mismatch and impairment of the diffusing capacity is a predictor of poor outcome, effective application of HFNC could have provided prognostic benefit to responders [2]. Even if data on its utilization in patients with AE-IPF are limited, HFNC has the potential to improve oxygenation in patients not responding to conventional oxygen delivery systems [2]. This may take place through a variety of mechanisms of action: first, it seems to provide better matching of gas flow in individuals generating a high inspiratory flow, as in the case of patients with IPF, by limiting the entrainment of room air during inspiration and ensuring higher FiO2 [2]. Second, although delivered through an open system, HFNC creates a small continuous positive airway pressure that may provide positive pulmonary distending pressure and alveolar recruitment, although intrinsic recruitability has been found to be low in patients with IPF [2].
HFNC improves the short-term prognosis of patients with AE-IPF in different ways. First of all it has an effect on hypoxemia and improves oxygenation, including in patients not responding to conventional oxygen therapy. Secondly, HFNC provides a PEEP-like effect which determines alveolar recruitment. In our specific case report we have tried to use the treatment of nasal cannula high flow in patients with acute exacerbation of IPF with higher levels of hypercapnia who refused NIV. The treatment was well tolerated and led to a reduction in work of breathing and a significant improvement of the clinical conditions and x-ray images of the chest. We achieved the best results in the systemic arterial blood gas values, passing from an acidic condition to stable with pH compensation. The patient was discharged from hospital in a stable clinical condition, indicating the HFNC therapy to be continued at home.




