Introduction
Morbidly adherent placenta (MAP) is usually associated with maternal morbidity and mortality [1]. The morbidly adherent placenta spectrum includes, placenta accreta when the placenta invades the endometrium beyond Nitabuch’s layer of decidua basalis, placenta increta when the placenta invades the myometrium, and placenta percreta when the placenta invades the uterine serosa [2].
The risk factors of MAP include previous caesarean section (CS), previous myomectomy, placenta previa, and damage of Nitabuch’s layer following intrauterine infection and/or endometrial curettage [3].
The incidence of MAP increases concomitantly with increased CSs rates [4]. The incidence of MAP is 3.3% in placenta previa without previous CS, while the incidence of MAP is 40% in placenta previa with history of 2 previous CSs [5].
The optimum planned delivery time for MAP is 34–35 weeks, following a course of corticosteroids and a multidisciplinary team approach [6].
An accurate diagnosis of MAP is essential to prepare the patient and caregivers for possible complications during delivery [7]. The ultrasound is a useful tool to diagnose MAP, with 77–93% sensitivity and 71–98% specificity [8–10]. Colour flow Doppler is more specific, with a 95% negative predictive value in diagnosing MAP, while magnetic resonance imaging (MRI) should be used for diagnosis of MAP cases with inconclusive sonographic findings [8, 9, 11].
The uterine preservation surgeries have been proposed for MAP in women who desire future fertility (i.e. young women with low parity) [12, 13]. The uterine preservation surgeries for MAP include the following: 1) uterine artery ligation; 2) placental-myometrial en bloc excision; and/or 3) IIA ligation [12, 13]. The complications of uterine preservation surgeries for MAP include severe postpartum haemorrhage, disseminated intravascular coagulopathy (DIC), intrauterine adhesion (IUAs), and/or intrauterine infection [14].
Hysteroscopy is the standard tool for uterine cavity evaluation. Office hysteroscopy is a quick, safe, and well-tolerated procedure, which allows diagnosis and treatment of intrauterine pathologies in the same outpatient setting [15, 16]. Therefore, this study was designed to evaluate the uterine cavity after uterine preservation surgeries for MAP.
Material and methods
Women ≥ 28 weeks pregnant with confirmed MAP by trans-abdominal and colour Doppler sonography, who desire future fertility and agreed for uterine preservation surgeries were included in this cohort study, which was conducted from January 2019 until January 2021, to evaluate the uterine cavity after uterine preservation surgeries for MAP.
Participants were included in this study after informed consent in accordance with the Helsinki declaration and after approval of the study by the institute Ethics Committee.
Women ≥ 28 weeks pregnant with suspected MAP were subjected to trans-abdominal sonography (TAS) followed by colour Doppler using a trans-abdominal convex probe (Alio 400, Toshiba, Japan) to confirm the diagnosis of MAP.
The diagnosis of MAP was suspected when women ≥ 28 weeks pregnant were diagnosed with placenta previa anterior covering the previous CS scar(s) using the TAS and confirmed by the diagnostic ultrasound and colour Doppler criteria of MAP [17, 18].
The trans-abdominal sonography diagnostic criteria of MAP include the following: 1) absence of the retroplacental hypoechoic zone with thinning of the retroplacental myometrial thickness (< 1 mm); 2) placental lacunae (irregular vascular spaces or moth-eaten placental appearance); 3) interruption of the uterine serosa-bladder interface, and 4) exophytic uterine mass invading the urinary bladder (in more severe MAP cases) [17, 18].
The colour Doppler diagnostic criteria of MAP include the following: 1) placental lacunae; 2) loss/disruption of the uterine serosa-bladder interface; 3) multiple vessels invading the uterine serosa-bladder interface [17, 18].
The uterine preservation surgeries for MAP include the following: 1) uterine artery ligation; 2) placental-myometrial en bloc excision; and/or 3) IIA ligation [12–13].
Uterine artery ligation
Uterine artery ligation was performed before the CS incision by holding the broad ligament on each side with the thumb finger anteriorly and index finger posteriorly, lifting its base below the site of the uterine incision; then the uterine artery was ligated on both sides using No. 1 VICRYL (Ethicon, Ethicon, Johnson & Johnson, USA). Uterine arteries were ligated with the surrounding myometrium to avoid uterine arteries injury [19].
Placental-myometrial en bloc excision
Placental-myometrial en bloc excision and repair was described by Palacios et al. [20]. It was used when ≤ 50% of the anterior uterine wall was invaded by the placenta, and it included resection of the invaded myometrium and the placenta en bloc after delivery of the foetus. Neovascularization bleeding was controlled by dissection and ligation. The myometrial defect was then repaired using mattress sutures horizontally [20].
Internal iliac artery ligation
While pulling the uterus to the counter side, the peritoneum over the psoas major muscle was opened between the round and infundibulopelvic ligaments. The peritoneal incision was then extended cranially to the level of the pelvic brim parallel to the infundibulopelvic ligament. The ureter was identified by holding the posterior leaf of the broad ligament with a blunt dissection towards the sacrum (the ureter is usually identified at the base of the broad ligament, medial to the IIA). After identification of the anterior and posterior division of the IIA, a right-angled clamp moved under the anterior division of IIA from the lateral to medial aspect, while its tip was directed upward (to avoid injury of the external iliac vein located on the inferola- teral part of IIA). When the tip of the right-angled clamp was seen on the medical aspect of the anterior division of IIA, the suture material (No. 1 VICRYL, Ethicon, Johnson & Johnson, USA) was grasped and the right-angled clamp pulled backward in the same direction. Finally, the suture material was tied around the anterior division of IIA [21].
Women ≥ 28 weeks pregnant with confirmed MAP, who desired future fertility (i.e. young women with low parity) and agreed for the uterine preservation surgeries were counselled regarding the complications of uterine conservative surgeries, which included severe postpartum haemorrhage, blood transfusion, DIC, IUAs, and/or intrauterine infection [14]. They were also counselled regarding the estimated risk of MAP recurrence in future pregnancies after the uterine preservation surgeries [22].
Participants managed by uterine preservation surgeries for MAP were evaluated 3–6 months after the surgeries using office hysteroscopies after exclusion of pelvic inflammatory disease, cervicitis, and/or pregnancy to evaluate the uterine cavity after uterine preservation surgeries for MAP.
Pelvic inflammatory disease was suspected with the minimal diagnostic clinical criteria (lower abdominal, adnexal, and cervical motion tenderness) and confirmed by the additional diagnostic criteria (> 38.3°C, cervical mucopurulent discharge, presence of numerous white blood cells [WBCs] in cervicovaginal fluid on saline microscopy, elevated ESR and C-reactive protein, and laboratory confirmation of cervical infection [either by N. gonorrhoea and/or C. trachomatis]) [23].
The diagnosis of cervicitis was based on the presence of visible purulent or mucopurulent endocervical exudate, endocervical bleeding easily induced by gentle touch of external cervical os and confirmed by the presence of > 10 WBCs/HPF in the cervicovaginal fluid on microscopic examination, and gram-negative intracellular diplococci in the endocervical exudate on Gram stain [23].
The pregnancy was confirmed in participants with amenorrhoea using a serum pregnancy test and either TAS or trans-vaginal sonography to detect an intrauterine gestational sac [24].
Office hysteroscopies were scheduled post-menstrual, 3–6 months after the uterine preservation surgeries in an outpatient setting by a senior gynaecologist blind to the conservative uterine surgeries done (to avoid potential bias), and without anaesthesia and/or antibiotics.
Participants were given oral non-steroidal anti-inflammatory/analgesic 30 mins before the office hysteroscopies. The participants were asked to empty the urinary bladder before the hysteroscopies and were positioned in the lithotomy position. The hysteroscopies were carried out in a standardized manner, using a 4-mm outer-diameter continuous flow Bettocchi hysteroscopy with 30° field of view (Karl-Storz Endoscopy, Netherlands) with normal saline as distension media [15]. Without a vaginal speculum the hysteroscopy was inserted vaginally towards the posterior fornix and slowly backwards to identify the uterine cervix. When the uterine cervix was identified, the hysteroscopy was carefully moved forward towards the cervical canal and then to the uterine cavity with the least possible trauma.
The uterine cavity was evaluated and explored for its shape and abnormality from the fundus downwards and from the left tubal ostia to the right one. The abnormalities detected during the hysteroscopic procedures were recorded and secondarily evaluated in a departmental meeting to detect the effect of uterine conservative surgeries done during management of MAP on the uterine cavity.
Participants were followed up after the office hysteroscopies for one year to detect the pregnancy outcome after the uterine preservation surgeries for MAP.
Finally, 40 women with MAP managed by uterine preservation surgeries and postoperative uterine cavity evaluation were included in this study to evaluate the uterine cavity after uterine preservation surgery for MAP (primary outcome). The secondary outcome measures the pregnancy outcome after the uterine preservation surgeries.
Statistical analysis
The collected data were statistically analysed using Statistical Package for Social Sciences version 20 (Chicago, IL, USA). Numerical variables were presented as mean and standard deviation (±SD), while categorical variables were presented as number (n) and percentage (%). The chi-square test (χ2) and Student’s t-test were used for analysis of qualitative and quantitative variables, respectively. P < 0.05 was considered significant.
Participants were included in this study after informed consent in accordance with Helsinki declaration, and after approval of the study by the institute Ethics Committee.
Results
Finally, 40 women with MAP managed by uterine preservation surgeries and postoperative uterine cavity evaluation were included in this study to evaluate the uterine cavity after uterine preservation surgeries for MAP. Table 1 shows the age, body mass index (BMI), parity, and number of previous CS of the studied women.
Table 1
The age, body mass index, parity, and number of previous caesarean section of studied women
The mean gestational age at the uterine preservation surgery for MAP was 36.4 ±1.4 weeks’ gestation. About 38 women (95%) had a normal menstrual pattern, while 2 women (5%) had postmenstrual spotting after the uterine preservation surgery for MAP (p = 0) (Table 2).
Table 2
The gestational age at the uterine preservation surgeries, menstrual pattern, hysteroscopic findings and pregnancy outcome after the uterine preservation surgeries
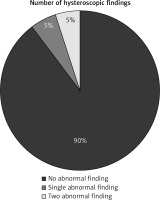
The hysteroscopic examination of uterine cavity after uterine preservation surgeries for MAP showed normal uterine cavity in 36 participants [36/40 (90%)], while it showed abnormal uterine cavity in 4 participants [4/40 (10%)], (p = 0).
The abnormal hysteroscopic findings were single abnormal hysteroscopic finding (endometrial polyp) in 2 participants [2/40 (5%)] and 2 abnormal hysteroscopic findings (incompletely healed scar with unilateral tubal ostial occlusion) in two participants [2/40 (5%)] (Fig. 1, Table 2).
The incidence of pregnancy after the uterine preservation surgeries for MAP was 7.5% (3/40); one of them had recurrent MAP managed by CS hysterectomy at subsequent delivery (Table 2).
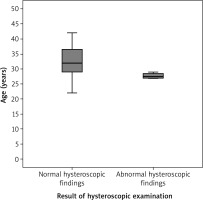
Participants with normal hysteroscopic findings after the uterine preservation surgeries were compared to those with abnormal hysteroscopic findings regarding their age, BMI, and gestational age at the uterine preservation surgery. Participants with abnormal hysteroscopic findings were significantly younger (27.5 ±0.9 years) compared to participants with normal hysteroscopic findings (32.0 ±2.9 years), (p = 0.03; 95% CI: 3.2, 4.5, 5.8) (Fig. 2, Table 3).
Table 3
The age, body mass index and gestational age at the uterine preservation surgeries in women with normal hysteroscopic findings compared to women with abnormal findings
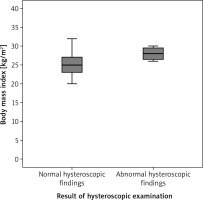
In addition, participants with abnormal hysteroscopic findings had significantly higher BMI (28.1 ± 0.5) compared to participants with normal hysteroscopic findings (25.2 ±1.7), (p = 0.03; 95%CI: –3.7, –2.9, –2.14) (Fig. 3, Table 3).
Discussion
The incidence of MAP increased concomitantly with increased CS rates [4]. The incidence of MAP was 3.3% in placenta previa without previous CS, while the incidence of MAP was 40% in placenta previa with history of previous 2 CSs [5].
Conservative uterine-sparing approaches for the management of MAP have been described to reduce the morbidity of peripartum hysterectomy and to preserve desired future fertility in selected women [14].
Uterine preservation surgeries have been proposed for MAP in women who desire future fertility (i.e. young women with low parity) [12, 13]. The uterine preservation surgeries for MAP include the following: 1) uterine artery ligation; 2) placental-myometrial en bloc excision; and/or 3) IIA ligation [12, 13].
The complications of uterine preservation surgery for MAP include severe postpartum haemorrhage, DIC, IUAs, and/or intrauterine infection [14]. Therefore, 40 women with MAP managed by uterine preservation surgeries and postoperative uterine cavity evaluation were included in this study to evaluate the uterine cavity after uterine preservation surgeries for MAP.
Hysteroscopic findings after uterine preservation surgeries for morbidly adherent placenta
The hysteroscopic examinations of the uterine cavity after uterine preservation surgeries for MAP showed normal uterine cavity in 36 participants [36/40 (90%)] and abnormal uterine cavity in 4 participants [4/40 (10%)].
The abnormal hysteroscopic findings were either single abnormal hysteroscopic finding (endometrial polyp) in 2 participants [2/40 (5%)] and 2 abnormal hysteroscopic findings (incompletely healed scar with unilateral tubal ostial occlusion) in 2 participants [2/40 (5%)].
About 38 women (95%) had normal menstrual pattern, while 2 women (5%) had postmenstrual spotting after the uterine preservation surgeries for MAP.
Twenty women were included in a study by Talamonte et al., who identified the hysteroscopic findings among women with prior CS, who presented with postmenstrual spotting [25].
Talamonte et al. found that the CS scars caused uterine cavity abnormality called pseudo-cavity (with variable depth) in 90% of the studied women (18/90), explaining the postmenstrual spotting [25].
Talamonte et al. observed dark brown blood inside the pseudo-cavity, explaining the postmenstrual spotting in 4 women as a single hysteroscopic finding (4/18) [25]. They found more than a single hysteroscopic finding in 50% (9/18) of studied women: pseudo-cavity and endometrial polyp in 4 women, pseudo-cavity and submucous myoma in 3 women, and granuloma inside the pseudo-cavity in 2 women [25].
In addition, Bij de Vaate et al., found the CS niche to be present in 56.0% of women with previous CS when examined by sonohysterography, and the CS niche was associated with postmenstrual spotting [26].
Factors associated with increased risk of caesarean section defects
In this study, the mean gestational age at uterine preservation surgeries for MAP was 36.4 ±1.4 weeks’ gestation. The studied participants with abnormal hysteroscopic findings were significantly younger with higher BMI (27.5 ±0.9 years and 28.1 ±0.5 kg/m2, respectively) compared to participants with normal hysteroscopic findings (32.0 ±2.9 years and 25.2 ±1.7 kg/m2, respectively) (p = 0.03 and 0.3, respectively).
Antila-Långsjö et al. concluded that maternal BMI, gestational diabetes, and previous CS were associated with increased risk for incomplete healing of the uterine incision [27].
Hayakawa et al. found that the increased gestational age at delivery, multiple pregnancies, and premature rupture of membranes were linked to increased risk and odds of wedge defects in CS scars [28].
A randomized controlled trial was conducted by Bennich et al. to investigate the effect of double-layer closure of CS uterine incision on residual myometrial thickness (RMT) [29]. They found that double-layer closure of the CS uterine incision did not increase RMT compared to single-layer closure [29].
The relationship between conservative uterine surgeries and Asherman’s syndrome
Intrauterine adhesion/Asherman’s syndrome may occur after the conservative treatment of placenta accreta and might be a direct cause of placenta accreta recurrence [30].
However, Sentilhes et al. reported severe IUAs and amenorrhoea in 8.3% (8/96) of women with MAP managed conservatively [22]. No IUAs were reported after the uterine preservation surgeries for MAP in this study, which can be explained by the small sample size of the cohort study.
To assess the effect of uterine preservation surgeries for MAP on the menstrual pattern, the uterine cavity should be evaluated in future larger studies.
Pregnancy outcome and recurrence of morbidly adherent placenta
The risk of recurrence of MAP probably depends on the type of conservative treatments used [12]. The incidence of pregnancy after the uterine preservation surgeries for MAP was 7.5% (3/40) in this study, and one of them had recurrent MAP managed by CS hysterectomy at subsequent delivery.
Sentilhes et al. studied 96 women with MAP managed conservatively and found that 34 of them were pregnant after the conservative management within 17.3 months mean time to conception (21 deliveries > 34 weeks, one ectopic pregnancy, 2 elective abortions, and 10 miscarriages) [22].
Sentilhes et al. reported recurrence of placenta accreta in 28.6% (6/21) and post-partum haemorrhage in 19.0% (4/21) of the studied cases [22].
This study was the first cohort study conducted to evaluate the uterine cavity after uterine preservation surgeries for MAP. The current study found the uterine preservation surgeries for MAP to have no effect on menstrual pattern, uterine cavity, and future fertility. The effect of uterine preservation surgeries for MAP on the menstrual pattern, uterine cavity, and future fertility should be confirmed in future larger studies. The small sample size and the women who refused to participate were the limitations faced during this study.
Conclusions
The uterine preservation surgeries for MAP in this study had no effect on the menstrual pattern, uterine cavity, and future fertility. The effect of uterine preservation surgeries for MAP on the menstrual pattern, uterine cavity, and future fertility should be evaluated in future larger studies.