Introduction
Cirrhosis is a major contributor to morbidity and mortality [1]. Decompensated cirrhosis, as evidenced by ascites, variceal bleeding, hepatic encephalopathy, and hepatocellular carcinoma, is a common presentation in patients requiring inpatient care for cirrhosis [2]. Patients with ascites have an increased risk of developing spontaneous bacterial peritonitis (SBP) even in the absence of any symptoms such as fever and abdominal pain [3]. Prompt analysis of ascitic fluid through diagnostic or therapeutic paracentesis by healthcare professionals is crucial for diagnosing SBP [4].
Performing paracentesis among hospitalized patients with ascites is an indicator for quality care in the management of patients with cirrhosis [5]. Furthermore, guidelines from the American Association for the Study of Liver Disease specify that paracentesis should be performed in patients admitted to the hospital with ascites regardless of the reason for admission [6]. However, there are no guidelines with regards to the optimal timing of performing paracentesis. Performing paracentesis has a greater diagnostic yield if done prior to antibiotic administration, as even a single dose of an antibiotic can cause specimen sterilization in most patients within six hours [6]. Moreover, early paracentesis in patients with ascites is associated with lower mortality rates as compared to cases where there is a delay in paracentesis [7].
The demographic variable of younger age is associated with delayed paracentesis [8]. There are mixed findings for female sex, with some reporting an association with delayed paracentesis [9] while others reported no such association [8]. The disease-related variables of an increased number of comorbidities [9] and high-risk patients [10] are associated with delayed paracentesis. Weekend admission is associated with delayed paracentesis [1, 11] while daytime vs. nighttime admission is not associated with delayed paracentesis [8]. Serum measurements are not associated with delayed paracentesis [8].
We are not aware of any literature on variables associated with antibiotic administration before or after performing paracentesis. We studied the association of demographic, disease-related, admission timing, and serum measurement variables with antibiotic administration before or after performing paracentesis.
Material and methods
Setting
This is a retrospective study of 137 consecutive patients admitted to a community hospital in New York City between March 2017 and February 2021 with a diagnosis of ascites secondary to cirrhosis. All adults aged 18 years and older were included. The study was conducted ethically after approval from the Institutional Review Board. A waiver of informed consent was obtained due to the retrospective nature of the study.
Variables
Demographic variables were age (years), sex (male/female), race/ethnicity (white, non-white), and body mass index (BMI; kg/m2). Disease-related variables were alcohol abuse, hepatitis C, too little fluid for paracentesis, and spontaneous bacterial peritonitis, all measured as no vs. yes. Hospital admission timing was day (7:00 AM to 6:59 PM) vs. night (7:00 PM to 6:59 AM). Serum measurements were hemoglobin (g/dl): abnormal ≤ 7 g/dl, international normalized ratio (INR): abnormal ≥ 2, and platelet count (× 103/mcl): abnormal ≤ 100 × 103/mcl, all measured as normal versus abnormal. Outcome variables were: 1) 12-hour paracentesis delay after initial encounter (no/yes) and 2) whether paracentesis was performed before antibiotic administration, after antibiotic administration, or not performed.
Statistical analysis
Mean and standard deviation were used to describe the continuous variables. Frequency and percentage were used to describe the categorical variables. Univariate analysis of variance was used to compare the continuous variables. Univariate analysis of either the Pearson chi square test or Fisher’s exact test when expected cell size < 5 was used to compare the categorical variables. All variables with a p-value of < 0.10 were included as predictor variables in a multinomial multivariate logistic regression analysis. IBM SPSS Statistics Version 28 (IBM Corporation, Armonk, New York, 2021) and Stata SE Version 17 (Stata, College Station, Texas, 2021) were used for the analyses. All p-values were two tailed with the alpha level for significance at p < 0.05.
Results
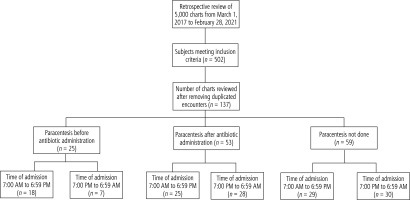
Regarding paracentesis, 18.2% (n = 25) of patients had paracentesis before antibiotic administration, 38.7% (n = 53) of patients had paracentesis after antibiotic administration, and in 43.1% (n = 59) paracentesis was not done (see Fig. 1). Regarding time of procedure, 36.5% (n = 50) of patients had paracentesis delayed for 12 or more hours after the initial encounter. Table 1 shows comparisons between the paracentesis groups. Female sex significantly differed (p = 0.003), the highest percentage being in the paracentesis after antibiotic administration group. Body mass index significantly differed, the highest mean being in the paracentesis before antibiotic administration group. Too little fluid for paracentesis significantly differed (p < 0.001), the highest percentage being in the paracentesis not done group. Time of admission from 7:00 PM to 6:59 AM had a p-value slightly above significance level (p = 0.096), the lowest percentage being in the paracentesis before antibiotic administration group. Platelet count had a p-value slightly above significance level, the highest percentage for abnormal platelet count being in the paracentesis not done group (p = 0.06). Time of admission from 7:00 PM to 6:59 AM significantly differed in analyses excluding the paracentesis not done group (p = 0.04) and also when combining the groups of paracentesis after antibiotic administration with paracentesis not done (p = 0.03), the lowest percentage being in the paracentesis before antibiotic administration group (data not shown).
Table 1
Comparisons between paracentesis groups
Table 2 shows that none of the comparisons between the time of paracentesis procedure groups significantly differed. Table 3 shows the multinomial multivariate logistic regression analysis. In analyses comparing paracentesis after antibiotic administration with the reference group of paracentesis before antibiotic administration, time of admission from 7:00 PM to 6:59 AM was significantly associated with increased relative risk for paracentesis after antibiotic administration (relative risk ratio [RRR] = 3.01, 95% CI: 1.02-8.85, p = 0.046). In analyses comparing paracentesis not done with the reference group of paracentesis before antibiotic administration, increased body mass index was significantly associated with decreased relative risk for paracentesis not done (RRR = 0.84, 95% CI: 0.74-0.96, p = 0.01).
Table 2
Comparisons between the time of paracentesis procedure groups
Table 3
Multinomial multivariate logistic regression analysis for paracentesis
Discussion
We did not find any significant difference in demographics, disease-related, hospital admission timing, or serum measurement variables according to a delay in paracentesis. There were two significant findings for paracentesis performance and timing of antibiotic administration. For those admitted at night, there was a significantly increased relative risk for paracentesis after antibiotic administration. Also, those with lower BMI had a significantly increased relative risk for paracentesis not done.
We did not find any significant association of demographic, disease-related, admission timing, or serum measurement variables with delay in paracentesis. Our findings differ from previous research in which younger age was associated with a delay in paracentesis [8]. A possible reason is that clinicians may delay paracentesis for those of younger age due to possible perceived healthiness among those of younger age. Also, the younger average age of our sample may not have been wide enough to show a significance pattern for younger age. Our lack of association for sex is similar to research in which no difference was found in timing of paracentesis based on patient sex [8]. A surprising finding was the lack of an association of disease-related variables with delay in paracentesis. This is contrary to previous research reporting that an increased number of comorbidities was associated with delayed paracentesis [9]. It is possible that there are other disease-related variables in addition to what we studied that are key to understanding a delay in paracentesis.
We found a significantly increased relative risk for paracentesis after antibiotic administration for those admitted at night. Previous studies showed a delay in paracentesis for patients admitted over the weekend [1, 11]. However, a previous study found no association between day versus night admission regarding delayed paracentesis [8]. Research with stroke patients reported greater delay in receiving thrombolysis for night admissions than day admissions [12]. Our finding for paracentesis is similar to this pattern of delays in necessary interventions for night admissions. We suggest that lower healthcare staffing levels at night delay necessary interventions. We also suggest that those working at night may avoid performing the procedure at night due to lower healthcare staffing levels to properly manage any adverse outcomes.
We found that patients with a lower BMI had an increased relative risk of paracentesis not done. To our knowledge, our study is the first to examine the association between BMI and the paracentesis procedure. We are unaware of any procedure, other than bariatric surgery, where lower BMI is a contraindication for performing the procedure [13]. Although there is no formal contraindication of lower BMI for not performing paracentesis, it is possible that there is a practical reason for this observation, in that many people who underwent paracentesis had ascites, contributing to the higher BMI.
We did not find any association of platelet count with performing paracentesis. Paracentesis can be safely performed without concern of hemorrhage in patients with a low platelet count indicating thrombocytopenia [14]. Also, platelet levels did not differ between cirrhosis patients with and those without spontaneous bacterial peritonitis [15]. Our findings are consistent with this pattern. We suggest that clinicians when evaluating platelet levels did not believe that there would be bacterial infection or post-procedure bleeding complications.
This study’s strength is the analysis of a well-known but often overlooked quality metric among patients with cirrhosis and ascites of the utilization of paracentesis. Our finding of the increased relative risk for paracentesis after antibiotic administration for those admitted at night highlights the potential disparity in lack of resources occurring in inpatient settings in hospitals at night. This study has some limitations. Early paracentesis plays an important role in diagnosing spontaneous bacterial peritonitis. As we had a very small number of patients with spontaneous bacterial peritonitis, our lack of an association may have occurred due to insufficient power for analyzing spontaneous bacterial peritonitis with timing of paracentesis ordering before or after antibiotic administration. Additionally, the diverse patient population in our community-based hospital may not be representative of other communities that may have a less diverse patient population. Future prospective studies on utilizing a strategy of an overnight resident/hospitalist run interventional team may show a different association of timely ascitic fluid drainage with paracentesis before antibiotic administration.
In conclusion, there was increased relative risk for performing paracentesis after antibiotic administration for patients admitted at night. We recommend ongoing resident and hospitalist training to maintain competency in bedside procedures such as paracentesis for patients with cirrhosis. Also, increased staffing or the presence of a resident/hospitalist led interventional team during night shifts may also help optimize the rates of timely paracentesis.