Dear Editor,
Viral encephalitis has a low incidence but high morbidity and mortality [1, 2]. On many occasions, it is difficult to diagnose due to the non-specificity of the condition, the number of possible differential diagnoses and the lack of resources that lead to a delay in its identification with the subsequent appearance of sequelae [3]. We pre-sent the case of a 48-year-old woman with a personal history of arterial hyper-tension (under home treatment with captopril 25 mg every 12 hours orally), dyslipidaemia (under treatment with atorvastatin 20 mg every 24 hours orally), type 1 diabetes mellitus (under treatment with a combination of metformin 50 mg and sitagliptin 1000 mg every 24 hours orally) and avascular necrosis of the femoral head (in treatment with a fentanyl patch 50 μg h–1 subcutaneously), who attended the Emergency Department of our Hospital Centre due to abdominal pain in the right hypochondrium, nausea, vomiting and fever of up to 38.5ºC, of 48 hours of evolution. After analytical controls (low prothrombin activity (56%), high urea (51 mg dL–1), altered liver profile (total bilirubin 5.5 mg dL–1, direct bilirubin 5.1 mg dL–1, AST 73U L–1, ALT 72 U L–1, gamma GT 91 U L–1, LDH 406 U L–1), metabolic acidosis (pH 7.20, bicarbonate 15 mmol L–1 and base excess –12 mmol L–1) and C-reactive protein 39.8 mg L–1), the rest of the normal controls and abdominal ultrasound, she was diagnosed with acute cholecystitis and admitted to the General Surgery ward for conservative treatment with antibiotics. Twelve hours after her stay in the hospital ward, she presented haemodynamic instability with symptoms of hypotension and neurological deterioration. Urgent cranial computer-aided tomography (CT) was performed, which was normal, and as the patient was wearing a fentanyl patch for pain control, opioid levels were requested. Due to elevated fentanyl levels and presumed toxicity, intravenous naloxone was given to the patient. After the change in treatment, she presented agitation that was interpreted as a possible picture of opiate deprivation, worsening her level of consciousness to a score on the Glasgow scale of 9, with the appearance of a new fever peak of 38.5ºC. The laboratory assessment revealed: anaemia, thrombocytopaenia, and elevation of lactate dehydrogenase (LDH) and liver enzymes. For all these reasons, the patient was admitted to the Intensive Care Unit (ICU). Upon her admission to the ICU, the patient’s respiratory and circulatory systems were stable. However, she was drowsy (Glasgow Coma Scale score of 11), with neck stiffness, she did not obey simple orders, and her verbal response was confusing. On physical examination, the only finding was pain on palpation of the right hypochondrium with a positive Murphy’s sign. Various complementary tests were carried out during her admission to the ICU, of which it is worth mentioning a polymerase chain reaction (PCR) study for type A influenza in a positive nasopharyngeal smear, a study of cerebrospinal fluid with biochemistry with mononuclear cellularity, the results being negative, detection of antibodies and PCR of the virus in cerebrospinal fluid. An imaging study by cranial CT and electroencephalogram was without findings of interest. The brain nuclear magnetic resonance (MRI) displayed signs compatible with haemorrhagic encephalitis [4, 5]. Twenty-four hours after admission to the ICU, the patient presented epileptic activity with generalized tonic-clonic movements, an impaired level of consciousness and worsening of respiratory function with significant arterial O2 desaturation. We performed urgent orotracheal intubation with subsequent invasive mechanical ventilation. The patient was administered analgosedation, as well as anticonvulsant treatment. Samples were taken for microbiological study (bronchial aspirate, blood cultures and cerebrospinal fluid by lumbar puncture), and the presence of influenza H1N1 virus in the respiratory samples was confirmed by PCR analysis. Therefore, the patient received oseltamivir at the dose of 75 mg every 12 hours through a nasogastric tube. Biochemical analysis of the cerebrospinal fluid revealed that the lymphocyte count was below 500 μL-1 and the protein concentration was below 100 mg dL–1 with the glucose level within a normal value range. In the imaging tests, the brain MRI detected: multiple hyperintense lesions and oedema in T2 (Figure 1) and flair of asymmetric location and involvement of the basal ganglia, the thalamus, the cortico-subcortical substance and the trunk of the brain. Encephalon in T1 and Swan sequences suggest a haemorrhagic component of the same, and also microbleeds were observed in the corticosubcortical junction of both hemispheres. All of them are images compatible with encephalitis of viral aetiology with a haemorrhagic component. After these findings and despite targeted treatment, the patient progressed poorly, with no response to successive attempts to withdraw deep sedation. Therefore, it was decided to add corticosteroids to the treatment (methylprednisolone at a dose of 200 mg every 24 hours, for five days, by intravenous bolus infusion, followed by prednisone at a dose of 15 mg every 24 hours orally, in a descending pattern for four weeks) and human immunoglobulin (initial dose 50 g intravenous bolus, followed by 12 g intravenous bolus once a month during hospitalisation). Due to poor tolerance of enteral nutrition, oral oseltamivir was replaced by intravenous infusion of zanamivir (dose of 300 mg every 12 hours, for five days). In the following days, progressive neurological improvement was observed, and after withdrawal of analgosedation the patient’s trachea was extubated and she was successfully weaned from mechanical ventilation. After 60 days of ICU stay, the patient fully recovered, with only minor punctual temporal and spatial disorientation, and altered sensitivity on the left side of her body.
FIGURE 1
Brain MRI in Swan sequence. Edema at the level of the cortex; the midline is preserved, there are no space-occupying intra- or extra-axial lesions
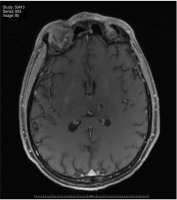
Influenza A virus stands out for producing mainly respiratory symptoms, with rare neurological or cardiac involvement [6, 7]. Most of the cases available in the literature on its impact on the central nervous system are collected in the paediatric population, with less than 5% of the cases being adult patients [8–10]. The neurological symptoms appear 7–10 days after the onset of the respiratory symptoms [11, 12], although in our case, the respiratory symptoms were not significant. The most common neurological symptom is a decreased level of consciousness, followed by seizures, visual disturbances, paralysis, or rigidity [13–15]. In our case, it began with a decreased level of consciousness and was followed by an isolated epileptic seizure. Regarding its diagnosis, the study of cerebrospinal fluid can show anything from normal to pleocytosis [16]. Cerebrospinal fluid PCR is positive in only 20% of cases described in the literature in which lumbar puncture was performed [17, 18]. And regarding the serotypes of the influenza virus, cases of encephalitis associated with HIN1 and H3N2 have been described [19]. Complementary tests such as head CT can be normal in more than half of the cases [20]. Brain MRI is the test with the highest sensitivity for its diagnosis [21, 22]. The findings in this imaging test are variable: oedema at the level of the cortex, hyperintense signals in the corpus callosum or lesions with a haemorrhagic component, as in the case we present [23, 24]. In our case, brain MRI was the test that guided us fundamentally towards its diagnosis. However, the mistake was made of not establishing differential diagnoses with hepatitis E virus or other viruses with tropism in the central and peripheral nervous system. Likewise, in the anamnesis, a more exhaustive epidemiological study was lacking in terms of recent trips or coexistence with animals. The treatment of this type of case is based mainly on life support and the administration of neuraminidase inhibitors, such as oseltamivir or zanamivir [25–27]. Likewise, cases have also been described in which corticosteroids have been used or plasma exchanges have been carried out. These last alternatives are based on the appearance of a possible abnormal immune response in the pathophysiology of this entity [28, 29]. In our case, different inhibitors were used, and corticosteroids and immunoglobulins were prescribed due to his poor evolution, without knowing which of the treatments, or perhaps the combination of all of them, helped to improve the patient’s evolution, being able to wake him up and disconnect him from the fan. As we mentioned at the beginning of this case report, a high mortality rate has been described in a series of similar cases, collecting up to 25% of patients with sequelae in the cases that survive [30]. However, studies with extensive follow-up to determine the degree of recovery have not yet been published [31]. Finally, it should be noted that seasonal flu can present with non-respiratory complications that we must diagnose and identify.




