Vitamin D is recognised as having two main functions in humans. In the classic function, vitamin D is responsible for extracellular calcium metabolism, namely intestinal absorption and musculoskele-tal milieu homeostasis [1–3]. In the pleiotropic (non-skeletal) function, it resembles a hormonal mechanism of action. Vitamin D binds to genomic sequences, known as vitamin D response elements, that are scattered in the body, and subsequently regulates gene expression. Specific vitamin D receptors are omnipresent in most human tissues. Vitamin D response elements are capable of subsequently modifying cellular processes such as prolife-ration, differentiation, apoptosis, angiogenesis, hormone secretion, and membrane stabilisation [3–9]. The above-mentioned modifications potentially influence physiological interactions such as anti-inflammatory processes, blood pressure regulation, glycaemic control, as well as the modification of innate and adaptive immunity [1, 3–9].
Vitamin D deficiency is very common in critically ill patients [9]. Severe deficiency is a risk factor for acute respiratory distress syndrome, acute kidney injury, multiorgan failure, and morbidity in critically ill septic shock patients [9–17].
The characteristic deficiency and decline in vitamin D serum concentrations shortly after admission have been observed in intensive care patients [9, 17]. Several studies have reported a relationship in critically ill patients between severe deficiency and mortality and length of ICU stay [12, 18–28]. However, most of these trials were retrospective, and the vitamin D kinetics was not measured. Although vitamin D deficiency is a potentially modifiable factor that can be corrected by intensive oral supplementation in the ICU [29], surprisingly there are only a few prospective observational or interventional clinical trials studying vitamin D serum concentration changes over time [9, 17, 26, 30–36]. Moreover, there are no scientific data regarding vitamin D serum concentration changes in multiorgan failure critically ill patients undergoing continuous renal replacement therapies.
The primary objective of this study was to assess the vitamin D serum levels in multiorgan failure critically ill patients undergoing regional citrate anti-coagulation continuous renal replacement therapies (study group) by performing periodic serum vitamin D measurements in short time intervals. The second objective was to compare the data obtained with the general intensive care population (control group, no renal replacement therapy group). We hypothesised that critically ill patients undergoing continuous renal replacement therapies are particularly prone to severe vitamin D deficiency during the course of critical illness.
METHODS
This was a prospective observational cohort study conducted from September 2015 to November 2018 in a single, 11-bed, mixed ICU. Written informed consent was obtained from the patients’ relatives. The study was approved by the Regional Ethics Committee (protocol number: 214/2015; date of approval: 25/03/2015), registered before the recruitment of participants (clinicaltrials.gov; Identifier: NCT02414386) and carried out according to the principles of the Declaration of Helsinki. The vitamin D serum concentration changes in a general population of intensive care patients (control group) were presented in our previous publication and used here as the historical control [9].
Control group
We included consecutive critically ill patients with vitamin D levels above 10 ng mL-1 at admission and with respiratory and circulatory failure. We defined respiratory failure as the need for invasive mechanical ventilation, and we defined circulatory failure as the need for inotrope and/or vasopressor administration [9].
Patients who met any of the following criteria were excluded: acute liver failure, acute kidney injury treated with renal replacement therapy, hyper-calcaemia at admission (total calcium plasma level > 10.6 mg dL-1, total ionised calcium plasma level > 1.35 mmoL L-1), parathyroid gland disease at admission, serum vitamin D level < 10 ng mL-1 at admission, end-stage renal disease, admission from another ICU or readmission, age under 18 years, or lack of consent from relatives. We established a cut-off value for serum vitamin D level of 10 ng mL-1 as extremely low. We assumed that below this level, vitamin D status assessment was pointless [9].
Study group
We included critically ill patients with vitamin D levels above 10 ng mL-1 at admission, with respiratory and circulatory failure and acute kidney injury treated with continuous renal replacement therapy by continuous veno-venous haemodiafiltration (CVVHDF), which was started no later than 48 hours after admission. CVVHDF was performed in each patient using regional citrate anticoagulation, Prismaflex system, and an ST150 set (Prismaflex, Gambro Lundia AB, Lund, Sweden). The CVVHDF dose was set between 30 and 40 mL kg-1 h-1.
Patients who met any of the following criteria were excluded: acute liver failure, hypercalcaemia at admission (total calcium plasma level > 10.6 mg dL-1, total ionised calcium plasma level > 1.35 mmoL L-1), parathyroid gland disease at admission, serum vitamin D level < 10 ng mL-1 at admission, end-stage renal disease, admission or readmission from another ICU, age under 18 years, or lack of consent from relatives. As in the control group, we established a cut-off value for serum vitamin D level of 10 ng mL-1 as extremely low.
Vitamin D (25-hydroxy-vitamin D) was measured in exactly the same way for both groups. Blood samples were taken from an arterial line or central venous line, or by direct peripheral venous puncture, and were collected in ethylenediaminetetraacetic acid (EDTA) tubes. Blood samples were protected from exposure to light, transported to the hospital laboratory within 30 minutes, centrifuged at 3500 rpm for 10 minutes, and processed by laboratory technicians. The vitamin D serum level was measured using an electrochemiluminescence binding assay on Cobas e411 or Cobas 6000 immunoassay analysers (Roche Diagnostics GmbH, Mannheim, Germany). The coefficient of variation (the amount of variability relative to the mean) of the method was estimated to be 0.8–5.8%.
Consecutive patients admitted to the ICU were assessed in terms of study participation (inclusion and exclusion criteria). In the majority of patients, the first vitamin D serum level was measured at the time of admission to the ICU. If the first vitamin D serum level was higher than 10 ng mL-1, the patient was included in the study. The first vitamin D measurements in the study group were performed before starting renal replacement therapies. The next set of vitamin D serum levels were taken in 12-hour time intervals (twice daily, at 6 a.m. and 6 p.m.). The minimum number of vitamin D measurements was four and the maximum was eight per patient [9].
All demographic data (date, name, hospital documentation number, sex, age, diagnosis at admission, comorbidities, Sequential Organ Failure Assessment Score [SOFA], and additional laboratory tests) were recorded in the hospital’s electronic database. After the recruitment process, patient data were extracted from the electronic database, the patient’s identification was blinded, and the data were transferred to the statistician for analysis [9].
Statistical methods
The quantitative variables were characterised by the arithmetic mean of standard deviation, median, and max/min (range). The qualitative variables were presented with the use of count and percentage. In order to check whether a quantitative variable derived from a population with a normal distribution, the W Shapiro-Wilk test was used. To prove the hypotheses on homogeneity of variances, the Leven (Brown-Forsythe) test was used. Statistical significance of differences between the two groups was tested with Student’s t-test or U Mann-Whitney test. The significance of differences between more than two groups was assessed with the F test. In the case of statistically significant differences between two groups, post hoc tests were used (Tukey test for F or Dunn for Friedman). c2 tests for independence were used for qualitative variables. In order to determine dependence, strength, and direction between variables, correlation analysis was used by determining the Pearson or Spearman correlation coefficients. In all the calculations a statistical significance level of a = 0.05 was used. Statistical analyses were performed using TIBCO (Software Inc., 2017), Statistica v. 13 (Palo Alto, CA, USA, 2017, http://statistica.io), and Excel.
RESULTS
A total of 1166 patients were evaluated for participation in the study. Reasons for exclusion in the control group were the following: no coexisting circulatory and respiratory failure, vitamin D measurement not performed, end-stage renal disease, vitamin D serum level below 10 ng mL-1, acute kidney injury treated with renal replacement therapy, admission from another ICU or readmission, acute liver failure, and age under 18 years [9]. Reasons for exclusion in the study group were the following: no coexisting circulatory, respiratory failure and acute kidney injury treated with CVVHDF, vitamin D measurement not performed, end-stage renal disease, vitamin D serum level below 10 ng mL-1, admission from another ICU or readmission, acute liver failure, and age under 18 years. Finally, 40 patients met the inclusion criteria, 20 patients were included in the control group, and 20 patients were included in the study group. The baseline demographics for both groups are shown in Table 1.
TABLE 1
Descriptive statistics of patients
Control group
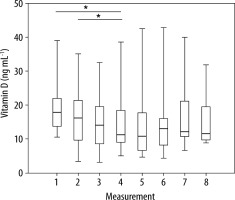
Figure 1 shows the distribution of vitamin D levels in the control group. The median vitamin D level decreased until the fourth measurement. This level stabilised around the fourth and fifth measurement, and then increased unevenly. In a sizable majority of time points, the distributions were skewed. The variability in observations was not constant over time, and there was no apparent trend [9].
FIGURE 1
The distribution of the level of vitamin D in the control group (*statistically significant differences)
The vector of fixed effect coefficients determines the shape of the curve that describes changes in the level of vitamin D for an average patient. For the first measurement, the average level was 18.57 ng L-1. This value changed over time, which is summarised by another two coefficients. Rather than considering a discrete rate of change in time, instead the change in the average level of vitamin D per small change of time could be investigated. This change can be derived by calculating the first derivative, which yields a linear function of time, i.e. –2.49 + 0.61 t. Substituting the time with the consecutive values (the first measurement at t = 0), the following change rates are obtained: –2.49, –1.88, –1.27, –0.66, –0.05, 0.56, 1.17, and 1.78. It is clear that the greatest decrease in the level of vitamin D was at the beginning. Subsequently, there was a minor decline, which could be considered a stabilisation; then, the final phase reflects a slight increase [9].
The analysis also demonstrates the importance of the patient effect, which is significant not only in terms of the average level of vitamin D but also the rate of change. This significance means that each patient’s intercept differs from the average value of 18.57 ng L-1 by the random effect of 7 ng L-1. There was a clear shift from an average-level curve to a patient-level curve. This variability is attributed to a different initial level of vitamin D. Similarly, each patient’s slope (rate of change) differed from the average value of –2.49 ng L-1 due to the random effect of 0.72 ng L-1 [9].
Study group
Figure 2 shows the distribution of the vitamin D level in the study group. Initially, the median vitamin D level decreased considerably and then stabilised around the third measurement. Unlike the control group, there was no subsequent increase. At most of the time points, the distributions were also skewed. The variability in observations was not constant over time, and there was no apparent trend.
FIGURE 2
The distribution of the level of vitamin D in the study group (*statistically significant differences)

The vector of fixed effect coefficients determines the shape of the curve that describes changes in the level of vitamin D for an average patient. For the first measurement, the average level was approximately 20 ng L-1. This value changed over time, which is summarised by another two coefficients. Rather than considering a discrete rate of change in time, the change in the average level of vitamin D per small change of time could be investigated. This change can be derived by calculating the first derivative, which yields a linear function of time, i.e. –8.45 t-2. Substituting the time for the consecutive values, the following change rates are obtained: –8.45, –2.11, –0.94, –0.53, –0.34, –0.23, –0.17, and –0.13. The greatest drop in the level of vitamin D was at the very beginning. Subsequently, there was a minor decline and, finally, a stabilisation.
The analysis also highlights the patient effect, which was significant not only in terms of the average level of vitamin D but also the rate of change. This finding means that each patient’s intercept differs from the average value of 20 ng L-1 by the random effect of 5.45 ng L-1. There was a shift from an average-level curve to a patient-level curve. This variability can be attributed to a different initial level of vitamin D. Similarly, each patient’s slope (rate of change) differs from the average value of –8.45 ng L-1 by the random effect of 7.43 ng L-1 at admission and 0.93 ng L-1 at the last measurement. Thus, the patient effect rapidly diminishes over time.
Comparative statistics
Table 2 shows the mean vitamin D levels for all measurements for both groups. There were no significant differences between the groups with respect to the level of vitamin D (P > 0.05). To compare both groups, analysis of mean values of the vitamin D levels in the study and control groups are depicted in Figure 3. Statistically significant correlations were found between the time of measurement and the level of vitamin D in the study (correlation coefficient R = –0.31, P = 0.0002) and control groups (correlation coefficient R = –0.18, P = 0.0341) (Figure 4). There were no significant differences between the groups with respect to correlation coefficient (P = 0.6886). Although the differences between groups gradually increased for the last three measurements, there was insufficient evidence to indicate that they were statistically significant.
TABLE 2
Tabular summary of mean/median values of the level of vitamin D in groups: study and control (mean, SD – standard deviation, minimum, maximum, and median)
DISCUSSION
A serum vitamin D concentration of less than 20 ng mL-1 is defined as a deficiency, a level between 20 and 30 ng mL-1 as an insufficiency, and a serum concentration of more than 30 ng mL-1 as a normal vitamin D level [7, 9, 37]. For our study, we defined severe deficiency as less than 10 ng mL-1. Our results reveal that critically ill patients are especially prone to initial severe vitamin D deficiency, and critical illness escalates this phenomenon.
We performed 200 initial vitamin D measurements during the study period (as part of the assessment for eligibility), 130 (65%) of which had serum vitamin D concentrations below 10 ng mL-1. We assume that the real severe deficiency rate could be even higher because for most patients excluded from the study, we did not perform measurements. A severe vitamin D deficiency is common in critically ill patients (estimated prevalence of 40–99%); however, the mechanism responsible has not been definitively identified [9, 18, 38, 39].
In our study, summary statistics of the vitamin D status in both groups revealed that the vitamin D serum levels were unstable during critical illness. The potential mechanisms responsible for this finding may be as follows: the serum vitamin D status mirrors the severity of illness, i.e. a hypoalbuminaemia, which is a typical feature of critical illness; low serum concentration of vitamin D binding protein during the course of critical illness; decreased synthesis of vitamin D binding protein; renal wasting of vitamin D; interstitial extravasation caused by increased vascular permeability; lack of sun exposure in the ICU; malnutrition; decreased renal production of 1,25(OH)D3; and increased tissue conversion of 25(OH)D3 to 1,25(OH)D3 [1, 9, 29].
In the control group, we observed a rapid decrease in vitamin D levels followed by stabilisation and then a small increase. We hypothesise that the observed trend could be consistent with the effect of therapy introduced after the patient’s admission to the ICU (clinical instability before treatment then stabilisation and improvement after the treatment implementation).
In the study group, the initial trends were similar to the control group, and stabilisation occurred around the third measurement. However, unlike the control group, there was no subsequent increase. We also hypothesise that the lack of a subsequent increase in the study group could have been influenced by CVVHDF (washout during convection process) or could be related to the fact that multiorgan failure patients with acute kidney injury are generally sicker and have an increased risk of mortality compared to patients without AKI.
Poor vitamin D status in critically ill patients raises the question of whether early and rapid supplementation in the initial phase of a critical illness could influence the outcome measures. In a randomised study, which assessed the effect of two doses of intramuscular cholecalciferol on serial serum vitamin D levels, Nair et al. found that correction of a vitamin D deficiency is possible in critically ill patients, but no statistically significant difference in mortality and hospital length of stay was observed [35]. In a randomised, placebo-controlled trial, Quraishi et al. investigated the changes in vitamin D status in septic ICU patients who were treated with a placebo versus cholecalciferol. They found that supplementation raises vitamin D serum concentrations in patients with sepsis and septic shock [34]. In the VITdAL-ICU study, Amrein et al. reported that the administration of a high oral dose of vitamin D versus placebo did not reduce the hospital length of stay, hospital mortality, and six-month mortality in the study group. However, the subgroup analysis revealed a trend towards lower hospital mortality in the severe vitamin D deficiency subgroup of patients in whom supplementation was performed [32].
A multicentre, randomised, placebo-controlled trial (VIOLET-NCT03096314) studying an early high-dose vitamin D supplementation in the critically ill revealed no apparent benefit [40]. The authors of the trial established a cut-off value of 20 ng mL-1 for vitamin D supplementation. However, it is known from the VITdAL-ICU study that a trend towards lower hospital mortality was observed when supplementation was performed exclusively in the severe vitamin D deficiency subgroup of patients (the cut-off value of less than 12 ng mL-1) [32].
Finally, another large, multicentre, randomised, placebo-controlled study (VITDALIZE-NCT03188796) investigating the relationship between intensive oral vitamin D supplementation and outcome in critically ill patients is underway. The recent European Society for Clinical Nutrition and Metabolism guidelines on clinical nutrition in the intensive care unit recommends a single high oral dose of 500,000 UL vitamin D within one week of admission in critically ill patients with vitamin D plasma levels below 12.5 ng mL-1 [29]. We still do not know if the recommended dose of 500,000 UL is suitable for multiorgan failure critically ill patients undergoing continuous renal replacement therapies. Based on the results of our study, a higher dose would probably be optimal in this group of patients.
Our study has three main limitations. The first is the small number of patients included. As previously mentioned, we established a cut-off value for serum vitamin D of more than 10 ng mL-1 as the inclusion criterion. We assumed that below this level, a vitamin D serum levels assessment was pointless. Given that severe hypovitaminosis D is very common in intensive care units, only 40 patients out of 1166 assessed for eligibility were included because of the strict inclusion criteria. The second limitation is the observational nature of the trial. A randomised trial studying the relationship between a high-dose oral vitamin D supplementation regimen in multiorgan failure critically ill patients undergoing continuous renal replacement therapies and outcome would be more informative. Finally, the study population was rather heterogeneous. However, our patients represent a typical intensive care milieu.
CONCLUSIONS
Multiorgan failure critically ill patients undergoing continuous renal replacement therapies are highly prone to severe vitamin D deficiency. We found that the vitamin D serum concentration decreases rapidly during the course of critical illness in these patients. However, we did not observe statistically significant differences between the renal replacement therapy group (study group) and general intensive care group (control group) with respect to the level of vitamin D (P > 0.05). Nonetheless, the differences between groups gradually increased for the last three measurements showing the probable general trend.






