Purpose
Cervical cancer remains a substantial global health concern, and is the fourth most frequently diagnosed cancer among women in the world [1]. The utilization of intra-cavitary high-dose-rate (HDR) brachytherapy using iridium-192 (192Ir) with external beam radiation plays a significant role in the treatment of advanced cervical cancer, demonstrating improvement of radiotherapeutic outcomes, such as enhanced target dose distribution and reduced number of adverse effects on nearby organs at risk (OARs).
The worldwide standard for accurate doses calculations in brachytherapy treatments is the American Association of Physicists in Medicine Task Group 43 (TG-43) protocol [2]. This protocol is specifically designed to compute dose-rate distributions around photon-emitting brachytherapy sources. In this approach, radiation interaction is modeled within an infinitely uniform water phantom, disregarding any internal or external variations in patient anatomy. This might affect the accuracy of dose calculations in areas near air or bone [3]. The limitations are that the formalism neglects the influence of tissue, bone, air gaps, and applicator material in dose calculation.
Previous research has indicated that TG-43 formalism tends to overestimate radiation doses in air cavities, and conversely, underestimate doses in high density substances [4]. Studies have proposed that for brachytherapy scenarios, such as prostate and cervix treatments, where tissues are relatively uniform, unshielded plastic applicators should be implanted, with no adjacent air pockets. Moreover, model-based dose calculation algorithms (MBDCAs) exhibit an average dosimetric impact of less than 5% compared with the TG-43 approach [5, 6].
The AAPM Radiation Therapy Committee Task Group 186 (TG-186) report provides updated guidelines for calculating and reporting doses in brachytherapy, aiming at overcoming the limitations of the current TG-43 method for calculating doses. Task Group 186 (TG-186) recommends clear guidance for adoption of MBDCAs in brachytherapy for accurate dose calculations, ensuring consistent practices. Notably, the inclusion of heterogeneity correction algorithms for tissue variations in brachytherapy is a recent development that enhances the precision of treatment planning systems (TPSs), unlike their use in external beam radiotherapy. This advancement comes from their ability to account for factors, such as air in the organs, materials of the applicators, and patient boundaries, resulting in more accurate calculations [4].
Advanced collapsed cone engine (ACE) is a convolution algorithm that does not rely solely on water-based calculations. It surpasses TG-43 by modelling radiation transport across various substances, including tissues, applicators, and air-tissue interfaces. This advancement significantly improves the accuracy of calculating dose delivered to patient [7, 8]. This method involves computing the dose delivered by primary, individually scattered, and multiple scattered photons separately, and then adding them together.
Therefore, the objective of the current study was to assess the dosimetric impact and compare the dose variations in plans generated in the ACE (TG-186) and TG-43 algorithms. This evaluation was conducted using tandem and ovoid intra-cavitary applicators for cervical cancer patients.
Material and methods
Patient selection and contouring
A retrospective study was conducted, involving 30 patients with locally advanced cervical cancer who received post-external beam brachytherapy treatment using tandem and ovoid Fletcher-Williamson special stainless-steel applicator (Elekta) [9]. The material, density, and element composition are shown in Table 1. Patients included in the study were categorized according to staging criteria outlined by the International Federation of Gynecology and Obstetrics, from stage IIB to IVA [10]. External beam radiotherapy (EBRT) was delivered with intensity-modulated volumetric arc technique using Varian UNIQUE™ Performance linear accelerator. A total dose of 45 Gy was prescribed to the pelvis, with 1.8 Gy per fraction. An additional 10 Gy was delivered with 2.2 Gy per fraction to the gross nodes using a simultaneously integrated boost. Each of the enrolled patients underwent computed tomography (CT)-based image-guided HDR brachytherapy (IGBT). Foley cathe-terization of the bladder was done, and inflated with 7 cm3 of air as a contrast before applicators insertions. Treatment was administered over three to four fractions, with each fraction delivering 7 Gy to high-risk clinical target volume (HR-CTV) over a period of 2 to 3 weeks. Typically, this treatment phase started in the final week of external beam radiation therapy (EBRT). All regions of interest (ROIs), such as HR-CTV, bladder, rectum, and sigmoid were contoured by the same radiation oncologist following the GYN GEC-ESTRO recommendations. All contours were re-checked by a second radiation oncologist in all patients [10]. Applicators were manually contoured using a pearl tool, guided by external dimensions provided by the vendor. Automatic contouring was employed for delineating air within patient organs and for outlining external body contour.
Treatment planning
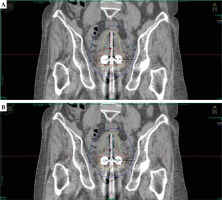
Treatment plans on TG-43 and ACE (TG-186) were made using Elekta Oncentra Brachy treatment planning system (OCB TPS) version 4.6.2. Initially, all plans were established based on the TG-43 formalism and subsequently normalized at point A, which was defined as a point 2 cm lateral to the central canal of the uterus and 2 cm up from the mucous membrane of the lateral fornix, in the axis of the uterus, according to the ICRU report 89 [11], followed by graphical optimization. Before performing ACE calculations, materials were assigned to delineated regions of interest (ROIs). Priority was given to overlapping structures, as indicated in Table 2. Material properties and associated mass densities were obtained from the AAPM TG-186 publication [4]. A set of 30 patients were re-planned using the TG-186 algorithm, maintaining the original dwell positions and dwell time without any adjustments, with 2.5 mm step size for all the plans. This process involved a step size of 2.5 mm for dwell positions for both the plans. A 3D dose grid setting of voxel size 110 × 150 × 150 mm was employed to delineate volume encompassed by 100% of prescribed isodose curve (IV100), and volume encompassed by 50% of prescribed isodose curve (IV50). A pear-shaped dose distribution of tandem and ovoid applicator for the TG-43 and TG-186 dose calculation algorithms are depicted in Figure 1.
Table 2
ROI properties used for ACE (TG-186) calculation from a case shown as example
[i] ROI – region of interest, ACE – advanced collapsed cone engine, HR-CTV – high-risk clinical target volume, HU – Hounsfield unit, CC priority – collapsed cone priority setting of the intersecting ROI by assigning numbers: 1 – highest priority, and 4 – lowest priority, N.A. – no intersection, DICOM – Digital Imaging and Communication in Medicine
Evaluation parameters
In this study, the following points, dosimetric parameters, and plan evaluation indices were evaluated according to the ICRU report 89 and other studies [11, 12].
Point-based parameters
The present study incorporated various point-based parameters, such as point A (located 2 cm lateral to the central canal of the uterus and 2 cm up from the mucous membrane of the lateral fornix) aligned with the uterus axis. Point B, located 5 cm from the midline at the level of point A, was also aligned with the uterine axis. BC represented the point at the center of 7 cm3 balloon within the bladder, while BP and RP were the bladder reference point and the rectum reference point, respectively [11].
Dose volume parameters
Dose volume parameters encompassed D90 and D100, which respectively represented the minimum dose delivered to 90% and 100% of planning target volume (PTV). Additionally, V100 and V150 denoted the volume of high-risk clinical target volume (HR-CTV) that received at least 100% and 150% of the prescribed dose. Moreover, the investigation included V200 and V300, indicating the volume of HR-CTV that received at least 200% and 300% of the prescribed dose. Furthermore, D0.1cm3 (Bladder), D0.1cm3 (Rectum), and D0.1cm3 (Sigmoid) represented the minimum dose to 0.1 cm3 volume of the bladder, rectum, and sigmoid. Moreover, the study examined D2cm3 (Bladder), D2cm3 (Rectum), and D2cm3 (Sigmoid), which reflected the minimum dose to 2 cm3 volume of the bladder, rectum, and sigmoid that received the maximum dose. Additionally, D10cm3 (Bladder), D10cm3 (Rectum), and D10cm3 (Sigmoid) parameters captured the dose to 10 cm3 volume of the bladder, rectum, and sigmoid [11, 12].
Plan indices
CI – coverage index, COIN – conformity index, DHI – dose homogeneity index, DNR – dose non-uniformity ratio, ODI – overdose index, EI – external volume index, GFB – gain factor bladder, GFR – gain factor rectum, GFS – gain factor sigmoid, GF – gain factor, PQI1 – plan quality index 1, PQI2 – plan quality index 2, PQS – plan quality score [12].
GI – gradient index:
Where IV50 is the volume (in cm3) covered by 50% of the prescribed isodose line, and IV100 is the volume (in cm3) covered by 100% of the prescribed isodose line.
Results
Point-based evaluations
The implementation of ACE (TG-186) in treatment plan calculations revealed a noteworthy reduction of 0.99% in dose at point A (averaged across both right and left points), and 1.36% dose reduction at point B (averaged across both left and right points), as compared with the TG-43 plans. These differences demonstrated statistical significance (p < 0.05). Particularly, a mean dose reduction of 9.67% was observed for point BC, 4.67% for point BP, and 3.14% for point RP (p < 0.001). The mean, standard deviation, and mean percentage difference are detailed in Table 3.
Table 3
TG-43 and ACE (TG-186) with p-values for point-based evaluation parameters
[i] Point A – averaged across both right and left points of point A, Point B – averaged across both right and left points of point B, BC – point at the center of 7 cm3 balloon in the bladder, BP – bladder reference point, RP – rectum reference point, * parameter follows non-normal distribution. All points were defined according to ICRU report 89
Dose volume evaluations
In the TG-43 plans, the mean D90 to HR-CTV was 109.90%, while in the TG-186 (ACE) plans, it was reduced to 107.02%. Similarly, for the mean D100 to the target volume, TG-43 was 69.89%, whereas TG-186 (ACE) exhibited 67.39%. In terms of the mean V100, TG-43 achieved 92.11%, while in TG-186 (ACE), it was reduced to 91.05%. As a result, the TG-186 (ACE) approach showed a mean dose reduction by 2.62%, 3.58%, and 1.15% for D90, D100, and V100 of HR-CTV, respectively, compared with the TG-43 algorithm. The TG-186 (ACE) plans demonstrated significant decreases in mean values for V150, V200, and V300 of HR-CTV compared with the TG-43 plans, showing 2.70%, 3.73%, and 4.25% reductions, respectively. In the TG-186 (ACE) plans, 2.31% reduction in doses to D0.1cm3 (Bladder), 2.74% reduction in doses to D2cm3 (Bladder), and 3.46% reduction in doses to D10cm3 (Bladder) were observed. Consequently, the values were 2.86%, 3.07%, and 2.57% lower for D0.1cm3 (Rectum), D2cm3 (Rectum), and D10cm3 (Rectum), respectively, in the TG-186 (ACE) plans compared with those of TG-43. The doses to D0.1cm3 (Sigmoid), D2cm3 (Sigmoid), and D10cm3 (Sigmoid) were also lower by 1.59%, 1.66%, and 2.23%, respectively, for the TG-186 (ACE) plans than for the TG-43 plans. All the dose volumetric parameters of HR-CTV and OARs exhibited statistical significance (p < 0.001), as detailed in Table 4.
Table 4
TG-43 and ACE (TG-186) with p-value for dosimetric parameters of HR-CTV and OARs
[i] ACE – advanced collapsed cone engine, D90 (HR-CTV) – dose to 90% of HR-CTV, D100 (HR-CTV) – dose to 100% of HR-CTV, V100 – volume of HR-CTV receiving 100% of the prescribed dose, V150 – volume of HR-CTV receiving 150% of the prescribed dose, V200 – volume of HR-CTV receiving 200% of the prescribed dose, V300 – volume of HR-CTV receiving 300% of the prescribed dose, D0.1cc (Bladder), D2cc (Bladder), and D10cc (Bladder) – minimum doses to 0.1 cc, 2 cc, and 10 cc volume of the bladder that receives the maximum dose; D0.1cc (Rectum), D2cc (Rectum), and D10cc (Rectum) – minimum dose to 0.1 cc, 2 cc, and 10 cc volume of the rectum that receives the maximum dose, D0.1cc (Sigmoid), D2cc (Sigmoid,) and D10cc (Sigmoid) – minimum dose to 0.1 cc, 2 cc, and 10 cc volume of the sigmoid that receives the maximum dose, IV100 – volume (in cm3) covered by 100% of the prescribed isodose line, IV50 – volume (in cm3) covered by 50% of the prescribed isodose line, * parameter follows non-normal distribution
Plan indices
The plans created in TG-43 and TG-186 (ACE) were subjected to assessment using various indices. The analysis revealed a marginal 0.02% increase in PQI 1 for TG-186 compared with TG-43, encompassing indices, such as CI, COIN, and DHI, primarily associated with HR-CTV, which did not reach statistical significance (p > 0.05). In contrast, TG-186 demonstrated lower values in PQI 2 compared with TG-43, and included DNR, ODI, and EI, focusing on normal tissues, which was statistically significant (p < 0.001). Overall, plan quality score (PQS) that encompassed other indices of the TG-186 (ACE) plans demonstrated a remarkable improvement of 13.47% when compared with the TG-43 plans. Furthermore, in the TG-186 (ACE) plans, gradient index (GI) demonstrated a reduction of 0.5% compared with the TG-43 plans. Notably, all indices showed statistical significance (p < 0.001), except for COIN, PQI 1, GFB, GFR, and GF (Table 5).
Table 5
TG-43 and ACE (TG-186) with p-values for plan evaluation indices
[i] ACE – advanced collapsed cone engine, CI – coverage index, COIN – conformity index, DHI – dose homogeneity index, DNR – dose non-uniformity ratio, ODI – overdose index, EI – external volume index, GFB – gain factor bladder, GFR – gain factor rectum, GFS – gain factor sigmoid, GF – gain factor, PQI1 – plan quality index 1, PQI2 – plan quality index 2, PQS – plan quality score, NV100 – normal tissue volume receiving 100% of the prescribed dose, * parameter with non-normal distribution
Discussion
The precision of brachytherapy treatment involves several aspects, such as accurate contouring of the target and organs at risk, precise placement of applicators during implantation, applicators reconstruction, and precise dose calculation. Despite the diverse aspects of precision, the ultimate objective remains unwavering, i.e., attaining a meticulous and accurate dose distribution.
In this study, the dosimetric disparities between two commercially available dose calculation algorithms were assessed. Dose variations between the TG-43 and TG-186 algorithms at point A and point B were marginal, with differences not exceeding 2%. While these distinctions might appear negligible, they become more pronounced when examining doses at the bladder and rectal reference points. Such notable differences can be attributed to the influence of air pockets within the respective organs.
Furthermore, dose volume parameters of target and OARs were evaluated. The D90, D100, and V100 for HR-CTV were 2.62%, 3.58%, and 1.15%, respectively; they were lower than in the TG-43, which indicate that the TG-43 dose algorithm overestimates the dose to HR-CTV. The dose reduction for TG-186 in volumetric parameters of OARs was less than 3.5% for D0.1cm3, D2cm3, and D10cm3 for the bladder, rectum, and sigmoid.
In a study by Mikell et al. [13], a retrospective evaluation was undertaken to examine the influence of heterogeneities on CT-based conventional Manchester system HDR 192Ir treatment plans for 26 patients. This assessment was carried out using Acuros BrachyVision version 8.8 software, and the authors reported dose to the water to medium. Their findings indicated that there were only minor differences at point A and B doses as well as in D2cm3 for the rectum, bladder, and sigmoid. Importantly, all dose parameters for individual patients showed differences in TG-43 values of less than 5%. In some cases, a subset of patients exhibited significant deviations exceeding 5%, in D2cm3 for the rectum and sigmoid. This deviation was observed when the treatment planning system assigned bone material to voxels within the radiopaque packing present in the vaginal region. In our patient cohort, we employed gauze pieces soaked in betadine solution to ensure clear visualization. It is also worth mentioning that the author’s study involved comparing the Aurous model-based dose calculation algorithm (MBDCA) with TG-43, while the current study focused on comparing the ACE MBDCA with TG-43. Jacob et al. [6] conducted a retrospective study involving ten patients who were treated with CT/MRI compatible tandem and ovoid HDR applicators. In their analysis, all plans were calculated using Oncentra treatment planning system version 4.3. The authors observed that the dose reduction in ACE (TG-186) when compared with TG-43, remained below 3% for various parameters, including point A, D90, D95, and D100 for HR-CTV as well as D0.1cm3, D1cm3, and D2cm3 for the bladder, rectum, and sigmoid.
In the present study, a comprehensive evaluation using various plan quality indices was conducted to gain an integral understanding of the overall quality of treatment plans. This approach provides a well-rounded assessment of the treatment plans. The overall PQS exhibited a notable improvement of 13.47% in ACE (TG-186) compared with TG-43. PQI 1, which comprised CI, COIN, and DHI, delivered an encompassing assessment of target coverage, conformity, and dose homogeneity within high-risk clinical target volume. Meanwhile PQI 2, composed of DNR, ODI, and EI, provided a comprehensive evaluation of target dose heterogeneity and potential hot spot volumes. The gain factor (GF) signified the overall improvement in plan quality, where D90 was considered in relation to D2cm3 values for the bladder, rectum, and sigmoid. While ACE (TG-186) plans showed only minor differences in PQI 1 and GF, with an increase of 4.06% in DHI, a substantial enhancement was evident in PQI 2 due to dose reduction in ODI and EI. Additionally, the V300 potential hot spot volume was observed to be 4.25% lower in the TG-186 plans when compared with the TG-43 plans. These findings play a pivotal role in the significant enhancement of PQS.
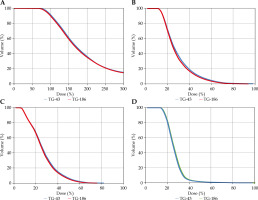
The gradient index (GI), a plan quality index closely aligned with the focus of this study, characterizes the dose fall-off in both the TG-43 and ACE (TG-186) plans. Figure 2 illustrates the isodose gradient concerning HR-CTV target, bladder, rectum, and sigmoid. Notably, GI was 0.53% lower in the ACE (TG-186) plans compared with the TG-43 plans. This difference can be attributed to dose attenuation resulting from applicator materials, air gaps, and soft tissues, as the TG-43 algorithm assumes homogeneity throughout the entire CT dataset, treating it as a uniform water phantom.
Fig. 2
Comparison of DVH plots of A) HR-CTV, B) bladder, C) rectum, and D) sigmoid for plans calculated using TG-43 and ACE (TG-186)
The American Association of Physicists in Medicine TG-186 report recommended the ongoing use of the TG-43 methodology for clinical dose calculations in brachytherapy, while concurrently conducting MBDCA calculations as a complementary approach [14, 15]. Utilizing model-based dose calculation algorithms (MBDCAs) for dose calculations and engaging in manual contouring of the applicators are time-consuming processes. However, these practices significantly enhance efficiency through precise dose predictions, effectively reducing the overestimation of doses to both the target volume and OARs.
Conclusions
The TG-43 algorithm tends to overestimate the dose to both the target and organs at risk in comparison with using the ACE (TG-186) algorithm for plans with identical dwell positions and dwell times. The differences in material composition between the tandem and ovoid applicator and surrounding tissues contribute to the overestimation observed in TG-43. This discrepancy arises from TG-43 assumption of patient body as a uniform water medium, which does not correctly reflect actual conditions. While TG-43 may exhibit dose overestimations, these variations remain below 5%. To enhance the precision of treatment delivery, it is advisable to report TG-43 doses in conjunction with ACE (TG-186), providing more comprehensive and accurate approach.