Purpose and rationale
The COVID-19 pandemic has adversely affected healthcare delivery systems throughout the world [1]. While the impact of this ongoing pandemic is largely asymmetric, with relatively developed countries bearing the largest burden of the virus, developing economies with less robust infrastructure are still bracing for the peak of the pandemic. Healthcare in general and cancer care particularly, are still evolving to adapt to this unprecedented challenge. Also, this crisis has caused the entire community to reconsider and revisit potential strategies for maintaining the safety of health caregivers, patients, and establishments. Cancer management poses a unique challenge in terms of a long duration of treatment, a need for regular monitoring, acute and delayed morbidities associated with aggressive therapies, etc., resulting in an enhanced risk of contracting infections [2]. Nevertheless, cancer treatment has been declared as an essential service that cannot be compromised during the pandemic [3,4]. Numerous guidelines and recommendations have been published by various agencies regarding the management of malignancies under these unusual circumstances [5,6].
The management of locally advanced cervical cancer presents specific challenges in this context. It is a rapidly proliferating cancer with high cure rates; therefore, postponement of treatment is an unviable option [7,8]. External beam chemo-radiotherapy, which is the standard treatment, is usually delivered in conventional fractions of 1.8 Gy to 2 Gy, over 4.5 to 5 weeks. Unlike other malignancies (e.g., prostate or lung), hypofractionated regimes to reduce the treatment time have not been proven to be efficacious in cervical cancer.
Brachytherapy for curative management of cervical cancer is indispensable, usually follows chemo-radiotherapy, and accounts for 50% of the radiation treatment [9]. Brachytherapy is delivered in multiple applications and fractions, with each application requiring placement of intracavitary/interstitial applicators under anesthesia, followed by imaging, planning, and treatment delivery. Therefore, brachytherapy may portend additional risks to the patient as well as the health caregivers in the time of pandemic. Hence, all processes related to brachytherapy, such as application, imaging, planning, and delivery described in various guidelines need to be adapted to a pandemic environment [10].
COVID-19 testing and triage
Before brachytherapy, along with routine pre-procedure work-up, the COVID-19 testing should be considered before each application. For patients who are hospitalized for the entire duration of brachytherapy, single testing before the first application may be considered sufficient, if appropriate precautions are assured during their hospital stay. This has the potential to reduce high-risk exposure to health caregivers, especially since early studies have identified the presence of coronavirus in urine and anal swabs in asymptomatic individuals apart from droplet borne spread [8,11]. However, the accuracy of testing modality used should be taken into account. For patients who were admitted and tested negative for COVID-19 during external beam radiotherapy, the extension of hospital admission to complete brachytherapy in a relatively safe environment may be considered to limit the need for repeated testing. However, in areas with a high incidence of COVID-19, tests may be repeated before each application, with an assumption that a negative test is ‘valid’ for 3 to 5 days. Figure 1 shows schema and possible workflow for cervical cancer patients and COVID testing for brachytherapy. Patients who test negative for COVID-19 should be treated with universal precautions, to reduce potential high-risk exposures to patients and the staff. For those who have suspicious symptoms, or for those with equivocal results on initial testing, deferring brachytherapy, isolation, and prompt (re) testing depending on institutional policies to confirm COVID-19 status may be a reasonable option before the further course of action is decided.
Fig. 1
Schema and possible workflow for cervical cancer patients and COVID-19 testing for brachytherapy
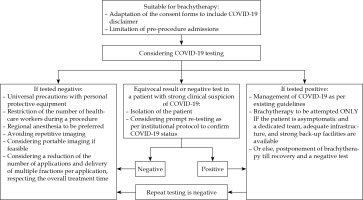
For patients who test positive, symptomatic or asymptomatic, the management strategy is more complicated and involves critical decisions for treatment of COVID-19, the continuation of treatment with brachytherapy, protection of health caregivers involved in brachytherapy processes and delivery, and other routine processes. Though evidence-based recommendations for the management of COVID-19 are available in the current international guidelines, questions regarding the management of cancer in patients who test positive before or during their cancer treatment remain mostly unanswered [12,13,14,15]. This mainly applies to the delivery of radiation therapy in cervical cancer, where overall treatment time is an important prognostic factor [10,16]. Some small, retrospective series have documented increased peri-operative mortality and morbidity in COVID-19 positive patients, who underwent major elective and emergency surgeries [14,15,17]. However, data on minor procedures including brachytherapy, is lacking at present. While the risks of continuing brachytherapy surely outweigh the benefits in symptomatic positive patients, strategies for treating asymptomatic positive patients need to be resource-customized. Employing a committed team, with a full gear of protective equipment, dedicated operating room and treatment machine, disinfection protocols for brachytherapy equipment, etc., may not be a feasible option for most centers [18]. In such circumstances, it is not unreasonable to wait for the recovery of patient and a negative COVID-19 result. Once a negative result is documented, re-testing may be done after a week (or as recommended by the local public health authorities) and brachytherapy can be considered accordingly. However, the number of applications ought to be limited and delivering multiple fractions per each application should be considered in such cases to compensate the unnecessary prolongation in overall treatment time. Maintaining a fine balance between the potential risks associated with such attempts and the risks related to undue prolongation of overall treatment time is imperative for achieving optimal outcomes. In principle, cancer care units should be well-prepared to confront such situations, with appropriately equipped ICU facilities and advanced precautions and measures.
Brachytherapy application, anesthesia, and imaging
Pre-operative parts preparation, medications, and consenting for brachytherapy procedures should include disclaimers related to the risk of COVID-19 infection. Pre-operative admissions may be avoided to reduce the risk of acquiring infection from asymptomatic patients in general wards; alternatively, admission in isolated rooms may be considered.
During the brachytherapy procedure, universal precautions with a personal protective equipment and appropriate disinfection practices should be absolutely adopted. Similarly, the number of health caregivers needed for the procedure should be restricted to the bare minimum [8]. Wherever applicable, the staff should be divided into groups, so that ongoing treatments can be sustained even if some of the members are exposed or infected.
Regional anesthesia should be preferred wherever feasible, to avoid aerosol-generating procedures associated with general anesthesia. However, achieving adequate perineal and pelvic muscle relaxation for vaginal packing assume even greater importance, to stabilize the application for longer durations and reduce the doses to organs at risk, especially if larger dose per fraction or multiple fractions per application are planned [16,19].
Repetitive imaging for treatment planning may pose additional risks to patients and health caregivers. The use of portable imaging in the brachytherapy treatment area to restrict multiple patient transfers should be implemented. If resources for imaging are limited, 2-dimensional planning can be considered, with point A-based prescriptions. For facilities practicing volumetric planning, the use of MRI planning can be restricted to the first application, mainly if the volume of residual disease is low and extensive reduction in further fractions is not anticipated. Alternatively, CT and trans-rectal ultrasound can also be used for volumetric planning in experienced facilities [10]. In centers where resources for planning are severely restricted, library plans available with the treatment planning system can be used based on applicator parameters, if the geometry is well-maintained and reproducible.
Dose, fractionation, and application
Delivery of multiple fractions per application, ranging from 2-5, has the potential to limit high-risk exposures to health caregivers and patients. However, such attempts should be based on sound and robust biological rationale, and supported by clinical evidence, so that appropriate balance can be achieved between high-risk exposures and disease/toxicity-related outcomes, without undue compromise of either. Needless to say, clinical factors like site and volume of residual disease, type of brachytherapy application, imaging modalities utilized, implant geometry, relative doses to the organs at risk, logistics of treatment facility, accessibility, expected compliance, etc., should be considered before adapting the recommended fractionation schedules [10]. Table 1 presents various fractionation schedules, number of applications, biological equivalent doses, and specific pros and cons remarks for each published [16,20,21,22].
Table 1
Various fractionation schedules, number of applications, biological equivalent doses, and specific pros and cons remarks for each published
| Schedule | No. of applications | EQD2 doses for tumor in Gy (α/β-10) for BT | Remarks | Reference |
|---|---|---|---|---|
| 5-6 Gy × 5-6 fractions | 5-6 | 37.5-40 | Increased risk of exposure due to large number of applications | [20] |
| 7 Gy × 4 fractions | 2 | 39.7 | Limited applications, without compromising the number of fractions | [16] |
| 8 Gy × 3 fractions | 3 | 36 | Overall treatment time may be prolonged if not interdigitated with EBRT | [22] |
| 9 Gy × 2 fractions | 2 | 28.5 | Compromised local control, especially for large volume residual | [20,21] |
| 9 Gy (on day 1) followed by 7 Gy × 2 fractions (on day 2, delivered at least 6 hours apart) | 1 | 34.1 | Risk of exposure reduced, logistic advantage; early outcomes encouraging Long-term outcomes awaited | Unpublished (CTRI/2017/ 03/008172, SIMBRACE study, Tata Memorial Hospital, Mumbai) |
The American Brachytherapy Society recommends five to six fractions of 5-6 Gy each, interdigitated with external beam radiation [20]. While this radio-biologically sound schedule and has a strong evidence base a strong evidence base for outcomes, it is limited by the number of necessary applications. International multicenter studies by the EMBRACE group have reported excellent local control and toxicity outcomes with four fractions of 7 Gy, delivered in 2 brachytherapy applications over 1 week, with 2 fractions per application in the majority [16,23]. The additional advantage of this regimen is that applications can be limited to two, without compromising the number of fractions. Another attractive option is to deliver high doses per fraction for each application. Retrospective and prospective studies have shown larger fraction sizes of 9 Gy in two applications to have inferior local control without a significant impact on overall survival [20,21]. However, such regimes can still be considered for small volume residual or elderly patients, after the assessment of risks and benefits.
Single application with multiple fractions has been practiced for pure interstitial template-based brachytherapy boost in post-operative recurrences, with reasonably good outcomes [24]. Currently, there have been attempts to deliver multiple fractions in a single application for intact cervical cancer, where MRI-based planning was used and 9 Gy was delivered on day 1, and two fractions of 7 Gy were delivered on day 2, after confirmatory CT imaging to review and address inter-fraction variations (CTRI/2017/03/008172, SIMBRACE study, Tata Memorial Hospital, Mumbai; abstract accepted for WCB 2020). Even though the feasibility of such regimen has been proven in that study and early disease-related outcomes are encouraging, long-term results regarding toxicity are awaited. Finally, at brachytherapy plan evaluation, if multiple applications are considered necessary to maintain a favorable therapeutic ratio, the brachytherapy applications can be repeated at shorter intervals (twice a week), on a case-by-case basis, to achieve and maintain the optimal overall treatment time.
In summary, the restriction of pre-operative admissions, mandatory pre-procedure testing for COVID-19, triaging patients and appropriate management according to test results, adherence to universal precautions, the use of regional anesthesia, reducing the number of applications, and delivering multiple fractions with each application, are some of the practical alternatives that can be explored for delivering essential brachytherapy treatments for cervical cancer patients, without unduly compromising the therapeutic ratio. Such measures should be individualized and customized to the severity of the pandemic in each region, to limit high-risk exposures to patients, health caregivers, and establishments/institutions in the future.



