Purpose
Colorectal adenocarcinoma is the third most common cancer worldwide and Europe’s second most common type of cancer, both by incidence and mortality [1, 2]. Surgical resection after pre-operative chemoradiation is the standard of care in treating advanced non-metastatic rectal cancer [3, 4]. A watch-and-wait protocol with active surveillance that has been practiced for nearly two decades, is an alternative to surgery for patients who achieved a clinical complete response (cCR) following neoadjuvant (chemo)radiotherapy [5-10]. This strategy aims to reduce the surgical harm resulting from increased post-operative morbidity, mortality, and adverse long-term functional outcomes, particularly in elderly, frail, and comorbid patients, many of whom are stoma averse [11-14].
However, the selection of patients for a watch-and-wait programme remains challenging, and the incidence of local tumor re-growth following a watch-and-wait approach ranges from 24% to 38% [8, 9, 14, 15]. Local tumor re-growth occurs most frequently within the first three years after treatment [9, 14, 15]. Approximately half of those patients who experience local tumor relapse, if left untreated, later present with refractory pain, fetid or bloody rectal discharge, a fungating rectal mass, and sometimes fistula formation, symptoms that tremendously impact patient’s quality of life [16, 17].
The established practice of care for managing local rectal cancer re-growth following (chemo)radiation is radical salvage surgery with total mesorectal excision, to improve local tumor control, local recurrence-free survival, and overall survival [15, 18]. However, this approach comes at the expense of organ preservation.
Contact X-ray brachytherapy (CXB) has been used in the treatment of rectal cancer for nearly 80 years, either as a sole treatment or in combination with external beam radiation or interstitial brachytherapy, to enhance local tumor control [19-25]. With rising bowel cancer incidence in the elderly [26] and higher surgical risks in those patients who have significant comorbidities or advanced age [11], CXB has been advocated as a non-surgical salvage option for individuals who experience local tumor re-growth after an initial complete clinical response following (chemo)radiation, to reduce the adverse effects of extirpative surgery.
Our study evaluated the effectiveness of CXB as a salvage treatment in rectal cancer patients who have experienced local tumor re-growth following a watch-and-wait programme. Oncological outcomes, symptom control, and tolerability were investigated. Additionally, the feasibility of performing salvage surgery for any residual disease or subsequent tumor re-growth following CXB was assessed.
Material and methods
Patient selection
The current study was approved by the institutional audit committee on May 3, 2022. As it was a retrospective study, the audit committee did not consider ethical approval was necessary. A total of 56 eligible patients were analyzed from the database at our center from 2009 to 2021. They had been referred to our Papillon unit from various colorectal cancer centers across the country. These individuals had experienced local rectal cancer re-growth during follow-up on an active surveillance programme, following an initial clinical complete/near complete response (cCR/nCR) after external beam (chemo)radiotherapy. Local tumor recurrence was confirmed by magnetic resonance imaging (MRI), endoscopy, and/or histology, and was defined as being either at an early- or more advanced-stage.
Initial patient management and surveillance
All patients had received initial neoadjuvant treatment, either as chemoradiation (45-50.4 Gy/25 fx./35 days), long-course (40-45 Gy/20 fx./28 days), or short-course (25 Gy/5 fx./5 days) radiotherapies, which was offered based on patient performance status and comorbidities at local colorectal cancer treatment centers. Those patients who achieved cCR or nCR were invited to participate in the watch-and-wait protocol. A cCR is defined as no palpable/visible tumor or only an erythematous ulcer/scar on digital rectal examination (DRE) and rectoscopy, and no observable residual tumor material/residual fibrosis only on MRI scans. The presence of small and smooth regular irregularities, including residual ulcer, small mucosal nodules, or minor mucosal abnormalities on DRE and rectoscopy as well as residual fibrosis but heterogeneous/irregular aspects and signal or regression of lymph nodes with no malignant enhancement features but with size of > 5 mm on MRI scans, is defined as nCR [27]. Patients were followed up closely with regular assessment using DRE, sigmoidoscopy, and MRI at their local centers, every 12 weeks in the first 2 years and every 6 months in the 3rd year, with subsequent endoscopic examination in the 4th and 5th years. If MRI and/or endoscopy suggested tumor re-growth, examination under anesthesia and biopsy was usually carried out to establish histological confirmation of tumor recurrence. Confirmed cases of local recurrence who were not suitable for or refused surgery were referred to our Papillon clinic for further evaluation and treatment.
Contact X-ray brachytherapy treatment for local re-growth
Before starting CXB treatment, patients were reviewed at the Papillon multi-disciplinary team (MDT) meeting for suitability and intent of treatment. Each patient was counselled on CXB not being a UK standard of care for local tumor re-growth, and the potential need for re-considering surgery if residual tumor/local re-growth will occur after CXB. Early-stage (ycT1/ycT2, ycN0) cases received CXB with curative intent, whilst bulky and more advanced stage (ycT3/ycT4, ycN0) cases received CXB with palliative intent for symptom control. Patients were informed about potential treatment side effects, and were requested to sign an informed consent form if they agreed with the proposed treatment plan.
Contact X-ray brachytherapy was administered on an outpatient basis using a Papillon-50 machine (50 kVp X-rays, HVL 0.64 Al, 2.7 mA; Ariane, Alfreton, UK), 30 Gy per fraction delivered 2 weeks apart, through a rectal treatment applicator (size 30, 25, or 22 mm) at a focal source surface distance of 29, 32, or 38 mm, respectively. Radiation was targeted straight onto the tumor with a 5 mm margin under direct vision. A total of 90-110 Gy (surface dose) was delivered in 3-4 fractions over 4-6 weeks. This dose falls to 50% at 5 mm depth, and 38% at 10 mm depth at each fraction. Therefore, Papillon is not suitable for bulky and infiltrative local re-growths.
Response assessment and outcome measures
After completion of CXB, patients were assessed every 3 months using DRE, sigmoidoscopy, and MRI scanning in the first two years and every 6 months in the third year according to the watch-and-wait protocol. Assessments occurred alternately at the local referring center and our Papillon clinic. Only endoscopy was performed in the 4th and 5th years for the responders following CXB. CT scans of the chest, abdomen, and pelvis were undertaken at 12, 24, and 36 months.
Outcomes were evaluated for the whole group of 56 patients and by sub-group analysis: 46 patients had early-stage tumor re-growth and were treated with curative intent, whereas 10 patients had more advanced stages of re-growth and received palliative CXB. The evaluated outcomes were local tumor control rate, symptom control, disease-free survival, and overall survival at one, three, and five years post-treatment. Outcomes of subsequent salvage surgery for residual/recurrent disease after CXB in terms of feasibility for R0 (clear resection margin)/R1 (free resection margin, 0-1 mm) resections, and post-CXB radiation side effects using common terminology criteria for adverse events (CTCAE) version 5.0 [28] were also assessed.
Statistical analysis
Quantitative data were expressed as the median with interquartile range (IQR). Categorical data were reported as the number of patients with percentages. Local tumor control rate was defined as the absence of recurrence after an initial clinical complete response after CXB treatment. Disease-free survival was calculated from the date of last CXB treatment to the date of R2 resection/recurrence after salvage surgery, local re-growth, distant metastasis, or last follow-up. Overall survival was assessed from the first date of diagnosis to the date of last follow-up or death from any cause. One-, three- and five-year survival rates were estimated using Kaplan-Meier curves. Associations between tumor characteristics and survival risks were analyzed using Cox proportional hazards models. Logistic regression was employed to evaluate influence of patient and tumor characteristics on local tumor control. A p-value < 0.05 was considered statistically significant. Statistical analyses were performed using R version 4.3.0.
Results
Patient, tumor, and treatment characteristics
Between 2009 and 2021, a total of 56 patients who had achieved cCR (80%) and nCR (20%) after initial (chemo)radiation and had developed local rectal re-growth during a watch-and-wait surveillance were analyzed. Forty-six patients (82%) had early-stage re-growth, and 10 (18%) had late-stage (ycT3/ycT4, ycN0). Among the early-stage patients, 26 were unfit for surgery due to their advanced age and/or comorbidities, and 20 refused surgery. Similarly, in the late-stage patients, 6 were unfit for surgery due to their advanced age (range, 78-90 years) and/or comorbidities, and 4 refused surgery, opting for palliative CXB. The median time to tumor re-growth following (chemo)radiation treatment was 13.3 months (IQR = 8-21), mainly occurring in the first and second years (Figure 1). Histological assessment was available for 45 (80%) patients, 27% showed high-grade dysplasia or were inconclusive, and 20% were diagnosed with DRE, endoscopy, and MRI without histology confirmation. Most patients (93%) received a total CXB dose of 90-110 Gy for salvage treatment, except for four cases who could not complete their treatment. Detailed demographic data are shown in Table 1.
Fig. 1
Proportion of patients who developed local re-growth over time after watch-and-wait protocol (cCR – clinical complete response; nCR – near complete response). Local re-growth occurred predominantly within 6 and 12 months in patients initially achieving nCR, while majority of those with cCR experienced local relapse slightly later, between 6 and 24 months
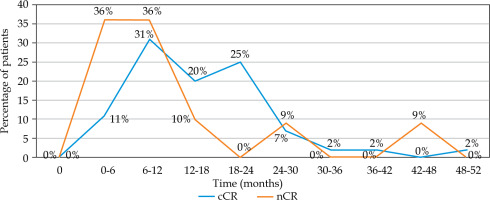
Table 1
Baseline demographic data of the whole cohort and sub-groups
* Two patients could not finish their full course of CXB treatment due to the COVID crisis; one patient had a stroke during treatment and did not receive a third fraction of CXB; the third fraction of CXB was discontinued in another patient because of complete disappearance of tumor only after the first fraction and very limited mobility of the patient
Clinical outcomes after contact X-ray brachytherapy
Whole group and sub-group analyses
The median follow-up was 37 months (IQR = 19-53). An overall initial clinical complete response at 6 months post-CXB was achieved in 32 (57%) patients. Eight of these patients (25%) experienced subsequent further local re-growth, without any nodal or distant metastases, while 24 (75%) achieved sustained local tumor control.
Among the 46 patients with early-stage tumor re-growth, 28 (61%) initially responded showing cCR/nCR at 6 months post-treatment. Sustained local control was achieved in 22 of these patients (79%), while 6 (21%) experienced further local re-growth over a median follow-up of 39 months (IQR = 13-66). All these six patients were managed with subsequent surgery.
In the group of 10 patients who had advanced-stage local re-growth, 4 (40%) showed cCR/nCR at the initial assessment following CXB. Two of these (50%) patients experienced sustained local tumor control, whereas 2 (50%) had further local re-growth over a median follow-up of 34 months (IQR = 10-57). Both of these patients were managed with subsequent surgeries.
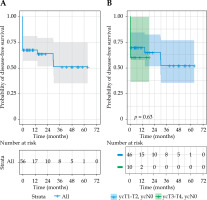
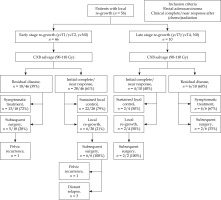
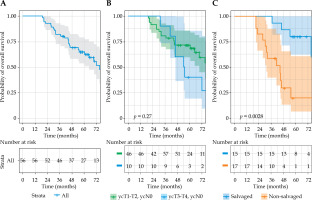
The overall disease-free survival was 69% at 1 year, and 51% at 3 and 5 years. The median overall survival was 75 months (IQR = 52-98), with rates of 100% at 1 year, 82% at 3 years, and 65% at 5 years. The overall survival (p = 0.27 [HR (95% CI): 1.6 (0.68-3.88%)]) and disease-free survival (p = 0.63 [HR (95% CI): 1.25 (0.42-3.78%)]) rates were not significantly different between early-stage and late-stage re-growth in unadjusted analysis. However, the late-stage re-growth group had a significantly higher risk of affecting the overall survival compared with those who had early-stage disease in the adjusted model (p = 0.03 [HR (95% CI): 5.46 (1.24-24.03%)]) (Figures 2 and 3). The overall clinical outcomes of the whole group are illustrated in a flow diagram (Figure 4). There was no statistically significant correlation between patient and tumor characteristics and local tumor control, as indicated by both univariable and multivariable analyses (Supplementary Table 1).
Fig. 2
Kaplan-Meier curves of overall survival for A) whole group, B) sub-groups based on yT-stage, and C) sub-groups based on having salvage surgery following contact X-ray brachytherapy failure
Symptom control
Common symptoms of patients at the time of local re-growth following initial neoadjuvant therapy in our study included rectal bleeding, discharge, local rectal pain, and altered bowel habits, as described in previous literature [16, 17]. These were observed in 11 patients (21%) in this study. CXB completely controlled rectal bleeding and discharge in those patients who experienced the above-mentioned symptoms. In three patients who developed rectal pain due to tumor re-growth, this pain was reduced from G2 to G1 in one patient, and was completely controlled in two other patients.
Subsequent treatment of local failures after contact X-ray brachytherapy
From the whole cohort of 56, 24 patients (43%) had residual disease following CXB. Salvage surgery was feasible in 7 of these patients, 3 patients ultimately opted against surgery, and the remaining 14 patients had high surgical risks due to their advanced age and/or multiple comorbidities. Consequently, all of these 17 patients received symptomatic and optimal supportive care only. Of the 7 patients who received salvage surgery, 5 had abdominal-peritoneal excision of the rectum (APER), one had low anterior resection (LAR), and one underwent pelvic exenteration. All achieved R0/R1 resection, except for one patient who had an R2 resection, and later developed further pelvic recurrence.
Following salvage CXB, 8 patients (25%) within the whole cohort experienced further local re-growth after achieving an initial clinical complete or near complete response. All were managed with subsequent salvage surgery; 7 had APER and one had a Hartmann’s procedure, all achieving R0/R1 resections. One patient had a further pelvic recurrence managed by pelvic exenteration. Three patients developed distant metastasis to sacral bones, liver, and lungs after salvage surgery.
The overall survival of patients who underwent salvage surgery (80% at 5 years) after CXB failure was significantly higher than that of patients who did not have a surgery (20% at 5 years) (p = 0.003 [HR (95% CI): 0.22 (0.08-0.64%)]) (Figure 3C).
Therapy-related side effects
Proctitis symptoms (erratic bowel habits) as acute reactions and late rectal bleeding occurred in only 10 patients (18%) after CXB. All these symptoms were self-limiting (CTCAE grade 1/2), and none of these patients required any intervention for their symptoms. Impaired anal sphincter function was not observed in any of the patients in our cohort.
Discussion
The standard of care for rectal cancer patients who develop local tumor re-growth during watch-and-wait active surveillance is salvage surgery, either as radical or local full-thickness excision [18, 29-31]. Due to the higher incidence of bowel cancer in older populations and higher surgical risks in older, frail, and comorbid patients [12, 31], many individuals are not suitable for extirpative surgery from the time of diagnosis to the occurrence of local relapse. In our study, more than half (54%) of patients were unfit for, while the others (46%) refused salvage surgery to avoid stoma formation in an attempt to maintain their good quality of life.
Local re-growth after initial (chemo)radiation was seen mostly within 6-12 months in patients who achieved nCR. By contrast, the majority of patients with cCR developed local relapses a little later than those with nCR (range, 6-24 months). This finding aligns with the time of local failure observed in other published studies on watch-and-wait approach [9, 15].
The local control rate by salvage surgery after watch-and-wait ranges from 83-97% in a series of published studies [8, 15, 32, 33]. In the current study, salvage treatment with CXB achieved local control in 24 (43%) patients overall and in 48% of patients who had early-stage re-growth, compared with 20% of patients who had more advanced tumor stages. CXB radiation penetrates only a few millimeters with a rapid dose fall-off, and delivers only 38% of the dose at 10 mm [24, 34]. It is, therefore, less likely to be effective in more advanced tumors. It is important to note that tumor response to CXB when treating local re-growth is considerably less than that observed when CXB is administered as an initial treatment for small residual cancer immediately following EBRT [25].
In the present study, further local relapse occurred within 5-13 months after CXB treatment, and the relapse rate was higher than that reported following salvage surgery with pathological complete response after watch-and-wait programme (25% vs. 3%) [35, 36].
The overall survival rates in the current study were comparable with those reported in patients undergoing surgical salvage after watch-and-wait [8, 15, 18, 26], and the highest rate was achieved in patients who could undergo salvage surgery following CXB failure. However, the disease-free survival rates were relatively lower. Due to patient frailty and comorbidities, 14 of 24 patients who had residual disease post-salvage CXB could not undergo surgery. Additionally, three others ultimately refused salvage surgery. However, these patients experienced stable disease without progressing to distant metastasis by the end of follow-up. This might have potentially contributed to the lower disease-free survival rate in this study.
Although other brachytherapy techniques, such as intraoperative radiotherapy (IORT) and high-dose-rate brachytherapy (HDR-BT) can also be used to treat rectal cancer, we are not aware of any published studies, which have addressed the efficacy of these modalities in the local re-growth setting after watch-and-wait protocol.
Contact X-ray brachytherapy appeared to reduce the symptoms associated with local tumor re-growth, significantly improving the quality of life of patients, particularly those who had more advanced tumor stages, even in palliative settings. Furthermore, the post-CXB side effects were manageable and all were self-limiting.
Insufficient data are available for comparing perioperative complications following salvage surgery, except for a prolonged operation time and a 10% incidence of surgical complications (Clavien-Dindo classification > III), which included a single anastomotic leak and a low conversion rate of 2% [27, 35]. Notably, all patients (100%) who had local relapses and 29% of those with residual disease after CXB achieved uncomplicated R0/R1 resections during subsequent salvage surgery. Unfortunately, however, two of these patients later developed pelvic recurrences.
The limitations of the current study include its retrospective, single-center, observational nature, and heterogeneity in patients’ demographics. Additionally, the disparity in tumor stage and size between the two sub-groups limits our ability to evaluate the genuine efficacy of salvage CXB. The inclusion of patients with more advanced stages of recurrent disease could have influenced the study outcomes.
Conclusions
In this cohort of patients who were unfit or refused surgery for their local rectal cancer re-growth following watch-and-wait (chemo)radiation, 48% achieved sustained tumor control following CXB alone, and 59% achieved remission following subsequent salvage surgery. This modality therefore offers a new treatment option for patients in a situation where other therapy options are not suitable or initial surgery is refused. Early-stage tumor re-growth responded better to CXB with minimal radiation toxicities and excellent symptom control. Disease-free and overall survival rates were acceptable, and delayed surgical salvage did not compromise long-term outcomes. Therefore, if there is uncertainty in patients with near complete responses who are under close surveillance in a watch-and-wait program, prompt referral to experienced Papillon centers is recommended in order to allow early assessment and treatment. This will give patients the best chance to respond and experience possible cures for their early re-growth, while there is minimal cancer burden.