Purpose
Cervical cancer is one of the most common malignant tumors in females in developing countries [1]. According to the National Comprehensive Cancer Network (NCCN) guidelines, surgery is the recommended treatment modality for early-stage cervical cancer [2]. However, cervical cancer patients may experience vaginal stump recurrence after undergoing radical hysterectomy [3]. He et al. reported that the 5-year recurrence rate of vaginal stump intra-epithelial neoplasia after hysterectomy in patients with stage I cervical cancer was 12.1% [4]. Brachytherapy plays an important role in the treatment of vaginal stump recurrence [5]. Post-operative recurrences of cervical cancer are a very heterogeneous group of diseases, and individual approach is required in many patients. Treatment strategies for vaginal recurrence of cervical cancer remain a complicated clinical issue, and surgical treatment is reportedly one of effective and relatively safe strategies for isolated diseases [6, 7].
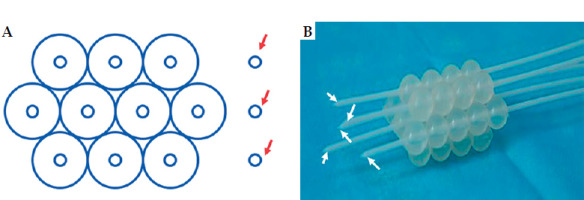
Several studies indicated that interstitial brachytherapy is an effective treatment approach for vaginal stump recurrence of cervical cancer [8-10]. Individualized 3D-printed applicator is another potential solution that requires well-trained 3D modeling and 3D printing technology support [11-14]. Freiburg flap applicator was designed for surface brachytherapy [15]. This flexible applicator can easily be shaped to fit curved surfaces; thus, they are creatively applied in other tumors [16, 17]. The Freiburg flap applicator can be used in various geometric combinations to be suitable for brachytherapy of vaginal stumps with vaginal stenosis (Figure 1). Interstitial needles can directly enter the tumor from the top of Freiburg flap applicator, and the arrangement between the needles conforms to the distribution principle of Paris system [18]. For larger tumors or unfavorable disease location, a combination of free-hand interstitial needles can achieve better target coverage and sparing of organs at risk (OARs). The purpose of this study was to report the initial application and preliminary clinical outcomes of using Freiburg flap as vaginal applicator with or without free-hand interstitial needles.
Fig. 1
Freiburg flap as vaginal applicator with or without free-hand interstitial needles. A) Schematic diagram of ten channels in Freiburg flap combined with three free-hand interstitial needles. B) A sample of Freiburg flap with seven channels and five needles. Red arrows point to three free-hand interstitial needles, white arrows point to five interstitial needles via Freiburg flap
Material and methods
Patients
Between September 2017 and January 2020, all patients with vaginal stump recurrence after radical hysterectomy of cervical cancer, treated with vaginal stump brachytherapy using Freiburg flap as vaginal applicator with or without free-hand interstitial needles, were retrospective analyzed.
The use of Freiburg flap as vaginal applicator mainly depends on the following aspects: 1. Freiburg flap has a certain degree of flexibility, is softer than 3D-printed template, and do not stimulate the vaginal mucosa. 2. The outer contour of Freiburg flap has uneven surfaces, instinctively possessing the ability to prevent from escaping from the vagina. 3. Freiburg flap can be assembled in different quantities, forming different diameters to adapt to patients with different vaginal elasticities. 4. The top of Freiburg flap is open, allowing interstitial needles to pass through. Compared with multi-channel vaginal cylinders, it can better conform to the thicker target volume of the stump. The current retrospective study was approved by the institutional review board of China-Japan Union Hospital of Jilin University (IRB No. 2022-KYYS047), and all patients signed informed consent form before enrollment.
External beam radiotherapy and brachytherapy
All patients received magnetic resonance imaging (MRI)-based image-guided brachytherapy. The use of Freiburg flap alone or combined with free-hand interstitial depended mainly on the thickness of vaginal wall invasion. This was decided before the procedure based on MRI before brachytherapy. Patients underwent detailed pre-operative evaluation and pre-planning to select the type of Freiburg flap applicator and the number of interstitial needles. The implantation of applicator and interstitial needles was carried out under anesthesia. During the process of implantation, trans-rectal ultrasound guidance was used to guide the direction and depth of the needles to ensure safety [19]. MRI was acquired with the applicator in situ using 3.0-T scanner (Siemens MAGNETOM Prisma, Erlangen, Germany). T2-weighted fast spin-echo sequences were obtained in axial, coronal, and sagittal planes, with 3-mm slice thickness. Also, T1-weighted spin-echo sequence was acquired in axial plane, to help reconstructing the channels of the applicator. MRIs were transferred to treatment planning system (Oncentra Brachy, Nucletron, Veenendaal, The Netherlands) for delineation of target volumes and OARs as well as for treatment plan. The delineation of target volumes and OARs was according to GEC-ESTRO recommendations [20, 21]. Due to the absence of cervix and uterus, high-risk clinical target volume (HR-CTV) included residual gross tumor volume (GTV), and areas on imaging and/or clinical examination that were of concern for harboring macroscopic pathologic disease. Intermediate-risk clinical target volume (IR-CTV) included a minimum initial GTV before external beam radiotherapy (EBRT), and encompassed a minimal safety margin of 0.5 cm added to HR-CTV.
Dwell positions were manually selected with 5 mm step size based on the target volume. Manual optimization combined with graphic optimization were employed to optimize the treatment plan. Planning aims were according to EMBRACE II research [22]. After evaluation of the treatment plan, patients were treated using iridium-192 (192Ir) remote afterloading system (Flexitron HDR, Nucletron, Veenendaal, The Netherlands).
Linear quadratic model was applied to calculate the equivalent dose in 2 Gy/fraction (EQD2). α/β value of organ at risk was considered 3, and α/β value of tumor tissue was considered 10.
Follow-up and statistical analysis
Follow-up was conducted once every 3 months in the first 2 years, and once every 6 months thereafter. Kaplan-Meier curves for local control and overall survival were generated with R software package (version 3.5.0, http://www.r-project.org ).
Results
Thirteen patients were enrolled, aged between 44 and 77 years, with a median age of 53 years. Two patients underwent post-operative radiation therapy two years and seven years before the study, respectively. One of the patients did not receive EBRT during the treatment of vaginal stump recurrence. In this patient, the median prescribed dose of EBRT was 45 Gy (range, 20-50 Gy), with 1.8-2 Gy per faction, using linear accelerator (EDGE, Varian, USA). Six of the thirteen patients received 1-4 cycles of concurrent chemotherapy with weekly cisplatin (40 mg/m2). The prescribed dose of brachytherapy was 28-39 Gy in 4-5 fractions, with weekly interval. The number of fractions using Freiburg flap as a vaginal applicator was 1-5, with a median of 3. The target volumes, OARs, and isodose lines of typical patients are shown in Figures 2 and 3. The patients and treatment characteristics are presented in Table 1. HR-CTV D90, IR-CTV D90, D2cc for the bladder, rectum, sigmoid, and small intestine are shown in Table 2.
Fig. 2
An example of Freiburg flap used as vaginal applicator without free-hand interstitial needles. A) Transverse section, B) sagittal section, C) coronal section, and D) 3D view. Solid lines in black, yellow, orange, red, purple, and blue represent isodose lines of 500%, 200%, 150%, 100%, 80%, and 50%, respectively, while dashed lines in red, green, blue, purple, yellow, and light blue represent HR-CTV, IR-CTV, bladder, rectum, small intestine, and sigmoid, respectively
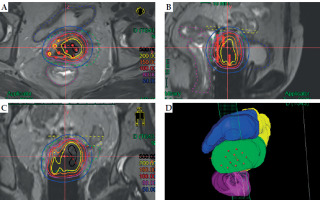
Fig. 3
An example of Freiburg flap used as vaginal applicator with free-hand interstitial needles. A) Transverse section, B) sagittal section, C) coronal section, and D) 3D view. Solid lines in black, yellow, orange, red, purple, and blue represent isodose lines of 500%, 200%, 150%, 100%, 80%, and 50%, respectively, while dashed lines in red, green, blue, purple, yellow, and light blue represent HR-CTV, IR-CTV, bladder, rectum, small intestine, and sigmoid, respectively. White arrows point to three free-hand interstitial needles
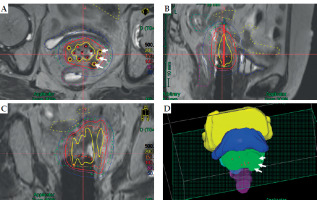
Table 1
Patient and treatment characteristics
[i] SCC – squamous cell carcinoma, ASCC – adenosquamous cell carcinoma, EBRT – external beam radiotherapy, BT – brachytherapy; *Two year ago, post-operative external beam radiotherapy was performed with a dose of 39.6 Gy in 22 fractions, **Seven years ago, post-operative external beam radiotherapy was performed with a dose of 50.4 Gy in 28 fractions
Table 2
Total equivalent dose in 2 Gy per fraction from external beam radiotherapy and brachytherapy
[i] HR-CTV – high risk clinical target volume, IR-CTV – intermediate risk clinical target volume, D90 – minimum doses delivered to 90% target volume, D2cc – minimum dose to 2 cm3 volume of OAR that received maximum dose; * Doses of primary pelvic radiation excluded in calculation of equivalent dose in 2 Gy per fraction
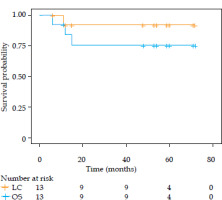
The median follow-up time was 54 months, (range, 6-72 months). The three-year local control and three-year overall survival were 90% and 75%, respectively (Figure 4).
Fig. 4
Local control and overall survival of enrolled patients
LC – local control, OS – overall survival
For early side effects, 9 patients (69.2%) experienced grade 1 side effects upon completion of the treatment. All these side effects disappeared without intervention 3 months after the treatment. In terms of late side effects, one patient had grade 3 rectal and urinary side effects, with rectum and bladder D2cc EQD2 of 69.8 and 76.7 Gy. Another patient developed grade 3 rectal side effect, with rectum D2cc EQD2 of 63.2 Gy. The response evaluation after treatment, survival status, and side effects are presented in Table 3. Until last follow-up, two patients (case 1 and case 7), who underwent re-irradiation were alive at 72 months and 60 months, respectively, and one patient experienced early G1 mucosal side effect.
Table 3
Evaluation of response after treatment, with survival status and side effects of patients
Discussion
Although local recurrence in cervical cancer patients after initial treatment may be a sub-clinical disseminated disease and may predict poor prognosis, many patients show long-term survival after salvage treatment. A Japanese study conducted by Ito et al. reported that the 10-year survival rate for recurrent cervical cancer of vaginal stump was 52% after comprehensive treatment [23]. Various treatment strategies, including loco-regional therapy (i.e., surgery and/or radiotherapy) and/or systemic therapy (i.e., chemotherapy and/or targeted therapy), can be considered to treat cervical cancer recurrence [24]. However, the optimal treatment for vaginal stump recurrence of cervical cancer is not clearly defined.
In our study, except for one patient who only received brachytherapy, all other patients were treated with EBRT (six patients with concurrent chemotherapy) and brachytherapy, to control recurrent tumors. This classic combination is a standardized treatment strategy in locally advanced cervical cancer, achieving good clinical outcomes and maintaining low toxicity [25]. Two 10-year studies have jointly revealed the irreplaceability of brachytherapy in radical radiotherapy for cervical cancer [26, 27]. The high-dose gradient in brachytherapy brings the possibility of delivering high-doses to target volumes while sparing OARs. However, this requires accurate distribution of dwell positions in key locations within the tumor, and higher constraints for the selection of applicators. Radical hysterectomy and post-operative radiotherapy may cause vaginal stenosis, fibrosis, and individual topography [28], making commercially available applicators unsuitable for adverse situations. In our study, the 3-year local control and 3-year overall survival rates were 90% and 75%, respectively. Chopra et al. performed EBRT combined with brachytherapy in 50 patients with post-surgical vaginal recurrences of cervical cancer, achieving 3-year local control and overall survival rates of 91% and 83%, respectively [29]. This result is in line with the current study. However, they adopted different applicator strategies. Martinez universal perineal interstitial template (MUPIT), small tandem with additional parametrial needles, and vaginal brachytherapy have been applied in different populations. This fully demonstrate the complexity and heterogeneity of patients with vaginal stump recurrence.
In our study, the re-applications, even in case of Freiburg flap plus free-hand interstitial needles, was mainly inspired by brachytherapy of cervical cancer. The fractionation strategy for brachytherapy in radical radiotherapy for cervical cancer mostly implements a weekly or twice weekly approach, even in interstitial brachytherapy [30, 31]. The use of fractionation strategy is mainly due to the higher toxicity with larger fraction sizes [32, 33]. On the other hand, patients may benefit from residual tumor shrinkage after high-dose of brachytherapy [34]. High-dose coverage in subsequent fractions will be higher and beneficial for tumor control. In our clinical practice, side effects related to applicator insertion are acceptable [35].
The number of channels in Freiburg flap depends on the cross-sectional area of the tumor in vaginal direction, rather than the volume of the tumor. In extreme cases, with a small cross-sectional area and large tumor volume, good high-dose coverage may be achieved with very few channels. When we used 10 channels in Freiburg flap, it seemed that the vaginal space was adequate for the placement of standard applicators. The reason for not using a standard applicator was due to the dosimetric advantages when Freiburg flap was used as a vaginal applicator compared with other competitive applicators. For example, the top of multi-channel cylinder applicator was closed, and was not beneficial for providing high-dose coverage to a thick vaginal stump. For ring intra-cavitary and interstitial (IC/IS) applicator or Utrecht IC/IS applicator, interstitial needles do not comply with the rules of Paris system, and the direction of the needles is at a certain angle to the vaginal axis, which is also not beneficial for dose coverage. In the selection of applicator, we followed the principle of prioritizing simplicity and availability in dose distribution. In pre-planning or first fraction, commercially available applicators are first considered. When dose distribution of commercially available applicator is insufficient to meet the planning aims, the applicator is replaced [36]. In the backup plan for replacing the applicator, the Freiburg flap is first considered, followed by more complex, 3D-printed applicators [12, 14].
Dose-effect relationships are objective and widely recognized in radical radiotherapy for cervical cancer, and often dominate treatment outcomes [37-39]. However, in the current study, the dose-effect relationships seem to failed in both tumor control and side effects. This may be related to the relatively small number of enrolled patients in this study. Therefore, further exploration is needed for the clinical application of Freiburg flap combined with free-hand interstitial needles. Two patients (15.3%) developed grade 3 side effects, suggesting that this technique carries certain risks.
In the current study, in addition to Freiburg flap needles, 19 fractions (46.3%) used free-hand interstitial as a supplement. Although free-hand interstitial is an important component of interstitial brachytherapy, it may introduce significant uncertainty in the position of the needle. However, the free-hand interstitial in our study was performed under real-time ultrasound guidance and performed by experienced physicians. This overcomes the shortcomings of traditional free-hand interstitial needle technique to ensure correct needle positioning, achieve high-dose coverage, and reduce positional change of the interstitial needle between two fractions. Compared with CT guidance, the advantage of real-time ultrasound guidance lies in its real-time guidance and the absence of radiation dose.
Pelvic recurrence following EBRT occurred in 20-40% of patients, making treatment selection difficult and lacking broadly recognized recommendations [40]. For patients who cannot tolerate surgery, re-irradiation may be a reasonable alternative. High-dose-rate (HDR) image-guided interstitial brachytherapy has attracted the attention of radiation oncologists as a notable option due to its superior dose distribution in target volumes, high-dose gradient at the edge of target volumes, and OARs-sparing ability [7, 8, 10, 41]. Our study provided a viable option for brachytherapy in patients with vaginal stump recurrence.
There are some limitations in the current study. The primary limitation is the limited number of enrolled patients. Moreover, not in all cases, the Freiburg flap applicator was used for every fraction. This weakens the connection between the treatment outcomes of this study and the Freiburg flap applicator. In addition, the free-hand interstitial needles technique involved in the study requires extensive experience of radiation oncologists, and is highly proficient in local anatomical structures, which pose certain obstacles to the promotion of this technology.
Conclusions
Based on our limited cohort, EBRT combined with 3D HDR brachytherapy using the Freiburg flap vaginal applicator with or without free-hand interstitial needles is one of the important treatment options for patients with vaginal stump recurrence of cervical cancer. Analyses of larger patient cohorts are required for better investigation of the correlation between dose and tumor control.



