Purpose
Rationale
Brachytherapy is an effective treatment modality in gynecological cancers. It offers a dosimetric advantage over external beam radiation therapy (EBRT) by delivering very high radiation doses to a target for optimal tumor control, with a steep dose fall-off away from sources, limiting dose to adjacent organs at risk (OARs) and potential radiation-related toxicities [1]. However, in gynecological cancers due to close anatomical proximity of the target to OARs, such as the rectum, adequate dose coverage of the tumor may result in excess dose to OARs beyond acceptable constraints.
A potential strategy to improve the therapeutic ratio is the use of spacers. A spacer can temporarily increase the distance between the target and OARs, allowing sufficient target dose coverage while reducing exposure to OARs [2]. The utilization of rectal spacers has been extensively studied in prostate cancer radiation, with randomized controlled trials confirming their safety and effectiveness [3, 4]. However, there are limited studies on the use of spacers in brachytherapy for gynecological cancers.
Objectives
This review aimed to evaluate the existing evidence on the use of injectable bio-absorbable spacers in brachytherapy for gynecological cancers.
The population, intervention, comparator, and outcomes (PICO) framework was employed, focusing on population (women undergoing brachytherapy for gynecological cancer), intervention (injectable bio-absorbable spacer), comparator, if applicable (no spacer), and outcomes (technique, safety, feasibility, failures, procedure-related complications, spacer assessment, dosimetry, toxicities, tumor outcomes, patient-reported outcomes, and cost-effectiveness).
Material and methods
Eligibility criteria
Inclusion and exclusion criteria used for the review are outlined below:
Inclusion criteria: 1. Studies reporting on the use of injectable bio-absorbable spacer in gynecological cancer brachytherapy; 2. Written in English language.
Exclusion criteria: 1. Use of non-injectable, non-bioabsorbable materials for spacer (e.g., balloon, mesh); 2. Use of open or more invasive procedure to insert a spacer (e.g., laparotomy or laparoscopy); 3. Non-spacer use of spacer/hydrogel material (e.g., fiducial marker, vaginal packing); 4. Non-gynecological cancer or site (e.g., head and neck, prostate, bladder, breast); 5. Unpublished manuscripts, conference abstracts, review articles, guidelines, editorials, or trial protocols; 6. Non-English language studies.
Information sources
A literature search was conducted in Medline, PubMed, Embase, and Cochrane Central Register of Controlled Trials. Dates of coverage were up to March 28, 2024 for Medline, PubMed, and Embase, and up to February 2024 for Cochrane Central Register of Controlled Trials.
Search strategy
The search strategy used was: gyne* or gynae* or cervix or cervical or endometrial or uterine or uterus or vagina* or female genital AND cancer* or carcinoma or neoplasm* or malignan* or tumour* or tumor* AND brachytherapy AND spacer or hydrogel or hyaluron* or SpaceOAR or polyethylene glycol or artificial ascites.
Selection process
Records from the search were imported into Covidence [5], a web-based collaboration software platform that streamlines the production of systematic and other literature reviews. Two reviewers (C.L., M.K.) screened each paper using title and abstract to identify articles to be retrieved for a full review. The reviewers assessed the retrieved articles for inclusion in the final review and subsequent data extraction. Consistencies and disagreements on study selection and data extraction were resolved by discussion and consensus between the reviewers.
Data collection process
Two reviewers (C.L., M.K.) extracted the following data from each article:
Study characteristics, such as patient number, study type, spacer material, clinical application (tumor site, e.g., cervix, vagina, mixed), funding, and conflict of interest.
Outcomes, such as insertion technique (with timing, imaging used for insertion, spacer location, volume inserted, and ease of insertion), success and reasons for failure, spacer insertion-related complications, spacer assessment (with separation distance, visibility score, symmetry score, dimensions, volume, imaging used to assess, and other quality metrics), dosimetry outcomes (including comparison with no spacer), toxicity outcomes, tumor outcomes, patient-reported outcomes, and cost-effectiveness.
Results
Study selection
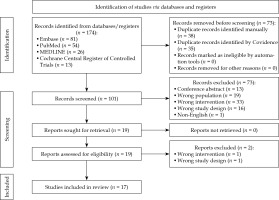
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow chart [6] is displayed in Figure 1. Of the 174 records identified, 73 duplicates were removed, 101 records were screened, and 19 were retrieved. Following discussion between the reviewers, one study using cadavers was included for the final review, as it assessed dosimetric impact following spacer insertion in gynecological cancer brachytherapy [7]. A total of 17 records were included in the final review with their data extracted.
Study characteristics
The studies which met the inclusion criteria are summarized in Table 1. There were seven case reports [8-14], five case series [15-19], three analytical/dosimetric papers [7, 20, 21], and two retrospective cohort studies [22, 23]. The number of patients within the studies ranged from 1 to 104, with the number of patients with spacer insertion ranging from 1 to 52 patients. The total number of patients across all studies was 312, of which 169 had spacer insertion. Twelve studies were from Japan [8, 11-14, 17-23], three studies from the United States [7, 10, 16], one study from India [9], and one study from Germany [15].
Table 1
Overview of the included studies
| Authors [Ref.] | Year | Country | Study type, number of patients (if comparison, number of patients with spacer) | Clinical application | Spacer material | Study objectives | Outcomes/conclusions |
|---|---|---|---|---|---|---|---|
| Marnitz et al. [15] | 2012 | Germany | Case series, n = 5 | Cervix | SpaceOARTM | To demonstrate the feasibility of hydrogel insertion in reducing rectal toxicity in cervical cancer brachytherapy | Spacer insertion is safe and feasible; 20 cc is not sufficient for rectal spacing in cervix cancer due to loose tissue of recto-vaginal septum and intra- and extra-peritoneal parts of the cervix |
| Kishi et al. [8] | 2012 | Japan | Case report, n = 1 | Vaginal recurrence of endometrial cancer | Suvenyl® | To report on hyaluronate gel injection into the para-rectal space for brachytherapy in vaginal stump recurrence of uterine cancer | Procedure completed in 45 minutes without complications; Achieved target dose D100% of 100.8 Gy EQD2, and recto-sigmoid D2cc of 65.7 Gy EQD2 |
| Viswanathan et al. [16] | 2013 | USA | Case series, n = 3 | Vaginal recurrence of gynecological cancers | DuraSeal® | To report on hydrogel placement in 3 patients with gynecological malignancies requiring re-irradiation with brachytherapy | Reduced rectal, sigmoid, and small bowel doses for first patient; Average rectal dose reduction of 11%; In two patients, average sigmoid dose reduction was 45% with spacer |
| Basu et al. [9] | 2016 | India | Case report, n = 1 | Cervix | Viscomet® | To demonstrate feasibility of hydrogel insertion into the recto-vaginal space in cervix cancer brachytherapy to reduce rectal toxicity | Reduced rectal D2cc and higher HR-CTV doses on fraction with spacer, compared with that without spacer; No adverse events |
| Damato et al. [7] | 2017 | USA | Analytical cadaver study, n = 5 | Cervix | TraceIT | To evaluate hydrogel injection between the cervix, rectum, and bladder in female cadavers compared with/and in addition to gauze packing for OARs sparing in cervical cancer brachytherapy | Decrease in rectal D2cc with hydrogel and gauze packing |
| Ahmed et al. [10] | 2019 | USA | Case report, n = 2 | Vaginal recurrence of gynecological cancers | TraceIT | To report on injection of an iodinated polyethylene glycol under CT guidance to separate the rectum in vaginal cuff | Lowered rectal D2cc and V30 with spacer; Grade 0 to 1 toxicity scores |
| Kashihara et al. [17] | 2019 | Japan | Case series, n = 36 | Mixed gynecological cancers | Suvenyl® | To report on initial experience of hyaluronic acid gel spacer in gynecological brachytherapy | No spacer-related adverse events; Rectum D2cc and D0.1cc significantly decreased; Increasing number of interstitial catheters and length from anal verge to most cranial point of 100% and 80% isodose lines negatively correlated with spacer dosimetric effect |
| Murakami et al. [18] | 2019 | Japan | Case series, n = 9 | Cervix | Suvenyl® | To report on hyaluronic acid gel injection into the vesico-vaginal septum in cervix cancer brachytherapy | Reduction in bladder D2cc with vesico-vaginal spacer; No severe spacer-related adverse effects |
| Murakami et al. [11] | 2019 | Japan | Case report, n = 1 | Cervix recurrence | Normal saline | To report on use of percutaneous administration of artificial ascites to separate adjacent bowel in recurrent cervix cancer re-irradiation | Reduced sigmoid D0.1cc with artificial ascites injection; No severe complications |
| Murakami et al. [22] | 2020 | Japan | Retrospective cohort study, n = 72 (spacer, n = 15) | Mixed gynecological cancers | Suvenyl® | To compare incidence of rectal bleeding in patients treated with vaginal cylinder brachytherapy with and without hyaluronic acid gel spacer | Spacer associated with reduced incidence of rectal bleeding; No procedure-related adverse events |
| Karube et al. [12] | 2020 | Japan | Case report, n = 1 | Vaginal recurrence of small cell cancer | 5% glucose | To report on artificial ascites infusion via the vaginal wall for interstitial gynecological brachytherapy case | Trans-vaginal artificial ascites through the pouch of Douglas was easy and effective in creating a space between the tumor target and intestine |
| Iijima et al. [20] | 2021 | Japan | Analytical study, n = 45 (spacer, n = 38) | Mixed gynecological cancers | Suvenyl® | To determine optimal location and volume of hyaluronic acid spacer into recto-vaginal or vesico-vaginal septum for OAR dose reduction | Spacer volume of 10 cm3 is sufficient, if the midpoint between applicator and OAR and cranio-caudal length covers active length of the applicator |
| Kobayashi et al. [23] | 2022 | Japan | Retrospective cohort study, n = 104 (spacer, n = 52) | Cervix | Suvenyl® | To compare HR-CTV, bladder D2cc, and rectum D2cc doses with and without hyaluronic acid gel spacer in cervical cancer brachytherapy | HR-CTV D90 dose was increased with spacer use, while keeping bladder and rectal doses within constraints |
| Takagawa et al. [13] | 2022 | Japan | Case report, n = 1 | Para-rectal recurrence of endometrial cancer | SpaceOARTM | To report on use of SpaceOARTM hydrogel spacer in recurrent endometrial cancer interstitial brachytherapy | Delivered CTV D90 of 97.3 Gy EQD2 with low rectum D2cc; No complications reported |
| Muramoto et al. [19] | 2023 | Japan | Case series, n = 5 | Cervix | MucoUp® | To report on the first use of MucoUp® as spacer in cervix cancer brachytherapy | Spacer associated with HR-CTV D90 to > 80 Gy and OAR dose within constraints; No spacer-related adverse events |
| Sakuramachi et al. [14] | 2023 | Japan | Case report, n = 1 | Cervix | Suvenyl® | To report on hydrogel spacer injection into meso-sigmoid for sigmoid protection in cervical cancer interstitial brachytherapy | Hydrogel spacer improved dose ratio of the sigmoid to HR-CTV D90 |
| Takatsu et al. [21] | 2024 | Japan | Analytical study, n = 20 (spacer, n = 10) | Cervix | MucoUp® | To assess dose escalation and reduction of fraction number in cervical cancer brachytherapy using a gel spacer | HR-CTV dose could be achieved with redu- ced number of fractions in the spacer group, while meeting OARs’ constraints |
Clinical application
The most common clinical application of spacers in gynecological cancer brachytherapy was for cervix cancer with eight reports (47%) found [9, 11, 14, 15, 18, 19, 21, 23]. In four studies, spacers were used in vaginal recurrences [8, 10, 12, 16], one paper reported on using spacer for para-rectal recurrence of endometrial cancer [13], and one article was a cadaver dosimetric study with brachytherapy applicators in situ [7]. The remaining three studies included patients with mixed gynecological malignancies [17, 20, 22].
Spacer material
The spacer materials used, and reabsorption time frames are summarized in Table 2. Five studies investigated polyethylene glycol hydrogels, of which two papers used SpaceOARTM (Boston Scientific Co., Marlborough, MA, USA) [13, 15], one study employed DuraSeal® (Integra LifeSciences Co., Plainsboro, NJ, USA) [16], and two studies reported on TraceIT (Boston Scientific Co., Marlborough, MA, USA) [7, 10]. Ten studies used hyaluronic acid-based products, of which one paper used Viscomet (Sun Pharmaceutical Industries Ltd., Mumbai, India) [9], seven studies (from the same center) utilized Suvenyl® (Chugai Pharmaceutical Co., Tokyo, Japan) [8, 14, 17, 18, 20, 22, 23], and two articles investigated MucoUp® (Boston Scientific Co., Marlborough, MA, USA) [19, 21]. One study used 5% glucose [12], and another study utilized normal saline [11].
Table 2
Spacer materials
| Spacer name | Material | Number of studies | Absorption time |
|---|---|---|---|
| SpaceOARTM [13, 15] | Polyethylene glycol | 2 | 4-6 months |
| DuraSeal® [16] | Polyethylene glycol | 1 | 4 weeks |
| TraceIT [7, 10] | Iodinated polyethylene glycol | 2 | Not specified; May still be visible at 3 months |
| Viscomet® [9] | Sodium hyaluronate | 1 | Not specified; Visible after 4 hours |
| Suvenyl® [8, 14, 17, 18, 20, 22, 23] | Sodium hyaluronate | 7 | 2-3 days |
| MucoUp® [19, 21] | Sodium hyaluronate | 2 | 2-3 days |
| 5% glucose [12] | 5% glucose in water | 1 | Not specified; Insertion required for every brachytherapy fraction |
| Normal saline [11] | 0.9% sodium chloride in water | 1 | Not specified |
Eleven studies reported on longer term visibility or reabsorption of the spacer material used. Takagawa et al. stated that SpaceOAR is expected to remain in place for 3 months with reabsorption by 6 months, and found that SpaceOAR was completely absorbed 4 months after spacer insertion on MRI [13]. TraceIT may still be seen on imaging for 3 months [7], and was visible on MRI 1-2 weeks after insertion [10]. Viscomet was found to reduce in volume from 9.3 cc to 7.3 cc after only 4 hours from insertion [9]. Due to their shorter reabsorption times, Suvenyl and MucoUp required spacer insertion for every brachytherapy fraction [14, 19, 21, 23]. Suvenyl was reported to last for 1-4 hours [8] and up to 2-3 days [14, 23], while MucoUp was absorbed within 2-3 days [19, 21]. Karube et al. acknowledged the uncertainty regarding the length of time using 5% glucose that would remain in the peritoneal cavity, but required insertion as a spacer with each brachytherapy fraction [12]. Viswanathan et al. expected DuraSeal to remain in place for 4 weeks before resorption, but no further imaging was performed for confirmation [16].
Timing of spacer insertion
Fifteen studies reported that spacer insertion was performed during the same procedure as brachytherapy applicator insertion [7-9, 11-14, 16-23]. In nine studies, spacer was inserted just prior to applicator insertion [9, 12-14, 16, 19, 20, 22, 23], while in three studies, the spacer was injected after applicator insertion [7, 11, 17]. Marnitz et al. inserted the spacer during week 3 of EBRT, in the same procedure as a Smit sleeve (Varian, Palo Alto, CA, USA) applicator insertion under general anesthesia [15]. Only one study by Ahmed et al. reported on a single patient with spacer insertion prior to EBRT and brachytherapy [10].
Location of spacer
Fifteen studies described insertion of a spacer into the recto-vaginal space [7-10, 12, 14-23]. Spacers were also inserted into the vesico-vaginal space [7, 12, 14, 17-21, 23], para-rectal space [8, 13], and intra-peritoneally for sigmoid or bowel spacing [11, 12, 14, 16].
Technique
Patients underwent spacer insertion under sedation, local, spinal, or general anesthesia. The most common approach reported for spacer insertion was in a lithotomy position [13-15, 19, 20, 22], whereas one study positioned patients prone [10]. Insertion point was mostly trans-vaginal [7, 12, 14-16, 18-23] or perineal [8, 10, 17] using an 18-21 gauge needle. Ahmed et al. also inserted through the medial right buttock into the distal anorectal fossa with a 22 gauge needle [10]. Murakami et al. used a percutaneous trans-abdominal approach, with ultrasound guidance for spacing intra-peritoneally [11]. The other intra-peritoneal spacer studies employed a trans-vaginal approach [12, 14, 16].
Insertion was carried out under trans-rectal ultrasound (TRUS) guidance for the majority (76%) of studies [7, 12-23]. Two studies used CT guidance [8, 10], whilst Basu et al. used an index finger per rectally to guide the needle without imaging for insertion [9]. Four studies reported hydro-dissection prior to spacer insertion, using either saline [19], spacer material [10, 17], or dilute contrast [10], with volumes between 1 and 3 cc.
The reported spacer volume injected in live patients ranged from 10 to 50 ml in the recto-vaginal space [9, 10, 15, 17, 21], 5-10 ml in the more limited vesico-vaginal space [18, 19, 21], 10-100 ml in the para-rectal space [8, 13], 5 ml in the meso-sigmoid space [14], and 500 ml in intra-peritoneal artificial ascites infusions [11, 12].
Only two studies reported on time taken for spacer insertion. Kishi et al. recorded approximately 45 minutes for their spacer injection procedure [8], whilst Muramoto et al. documented approximately 15 minutes for both bladder and rectal spacing [19].
Ease of insertion
Three studies reported on ease of spacer assembly, localization, or injection. Ahmed et al. reported that TraceIT was selected as a spacer material for ease of use, radio-opacity, availability as a pre-mixed single-syringe solution, and absence of time constraints during injection [10]. Damato et al. in their cadaver study reported variable ease, with some injections requiring significant force [7]. Kishi et al. indicated that peri-vesical and para-rectal space adhesions in post-surgical settings may lead to challenges in organ separation [8].
Failures
Four studies discussed failed spacer injections or potential reasons. Marnitz et al. suggested that recto-vaginal spacing with 20 cc was not useful for rectal separation from the cervix for two reasons. Firstly, the authors proposed that the capacious recto-vaginal space required more spacer volume, and secondly, they observed that effective separation could only be achieved in the extra-peritoneal part of the cervix [15].
Damato et al. found that insufficient recto-vaginal spacing inferior to the applicator allowed the rectum to move closer to higher dose areas, resulting in an increase in rectal D2cc in some cases, when compared with packing alone [7]. In addition, they reported that in one cadaver, the initial insertion had an “unsatisfactory distribution” as subsequent injections in the cadaver stayed in place; however, there was no further description [7].
Iijima et al. stated there were cases where hydro-dissection with saline in the vesico-vaginal space was unsuccessful, and therefore spacer insertion was abandoned. This was attributed to greater challenges with spacer insertion into the thinner vesico-vaginal space compared with recto-vaginal space, particularly during the initial learning phase [20]. Sakuramachi et al. reported failure of artificial ascites to separate their target from the sigmoid, likely related to re-distribution of saline within a short time [14].
Procedure-related complications
There were no severe procedure-related complications reported in any of the studies [7-15, 17-20, 22, 23]. Minor bleeding from the needle puncture site [18, 22] and small discomfort not requiring treatment [17] were observed.
Spacer assessment
Imaging modality used to assess spacer position was generally the same as that used for image-guided brachytherapy. The most common modality was CT, used in 16 studies [7-15, 17-23]. Five studies employed MRI, either alone or with CT [7, 10, 13, 15, 16].
Seven studies reported separation measurements of OARs from the target. The rectal separation values achieved were 5-19 mm [19], 7-26 mm [15], 16-20 mm [10], an average of 11 mm [9], and more than 15 mm [8]. Muramoto et al. reported 5-10 mm bladder separation with median 7 mm [19], while Viswanathan et al. showed small bowel separation of greater than 1 cm [16].
Two studies described spacer length measurements. In Marnitz et al. study, spacer length ranged between 18 and 38 mm with median of 32 mm in 5 patients [15], whilst Basu et al. reported a length of 5 cm [9].
There were no visibility scores in any of the studies. Damato et al. stated there was “good visibility” of spacer on both CT and MRI [7], and Marnitz et al. stated the spacer was “clearly visible” on MRI [15]. There were no scores of symmetry reported in any of the studies.
Damato et al. analyzed shifts of the superior and inferior borders of rectal spacer in cadavers with gel only and gel with packing. They noted shifts less than 1 cm, with a mean time between CT and MRI scans of 65 minutes (range, 60-71 minutes) and 41.4 minutes (range, 37-47 minutes) for gel only and gel with packing scenarios, respectively [7].
Kashihara et al. defined Lcranial100% and Lcranial80% as the length from the anal verge to the most cranial point, at which the 100% and 80% isodoses, respectively, crossed the rectum and found these correlated negatively with rectal dosimetric effect. Specifically, the more cranial these high isodoses overlapped with the rectum, the less effective the rectal spacer was on dosimetry [17].
Iijima et al. analyzed optimal location, shape, and volume of spacer injection into the recto-vaginal or vesico-vaginal spaces for effective dose reduction to OARs in patients treated with vaginal cylinder intra-cavitary brachytherapy, and suggested that optimal spacer shape was more effective for OARs dose reduction than increasing volume [20]. The authors used center of gravity positions obtained from the Eclipse scripting application programming interface (Varian, Palo Alto, CA, USA), and calculated midpoints between the gravity positions of applicator and OARs to assess injection location. On the rectal side, they found that spacer injection within ±5 mm and ±10 mm from the midpoint in the lateral-medial and cranial-caudal directions, respectively, larger spacer volume, larger cranio-caudal length, and larger ventro-dorsal thickness measurements were associated with lower rectal D2cc. For the bladder side, they correlated spacer injection within ±2.5 mm in the lateral-medial direction from the midpoint, larger spacer volume, and larger ventro-dorsal thickness with lower bladder D2cc. They proposed that a spacer volume of 10 cc injected on the midpoint between the cylinder and OARs, with cranio-caudal length extending the active length of the applicator, was sufficient to provide OARs dose reduction.
Dosimetry
Recto-vaginal spacer
Nine studies compared rectal dosimetry with and without the presence of a recto-vaginal spacer [7-10, 16, 17, 21-23]. Of these, eight studies showed a reduction in rectal doses with the use of a recto-vaginal spacer [7-10, 16, 17, 21, 22]. Rectal D2cc was evaluated in eight studies [7-10, 17, 21-23], and the results are summarized in Table 3.
Table 3
Studies comparing rectal D2cc with and without recto-vaginal spacer
| Study | Rectal D2cc (Gy) | Cumulative EBRT + brachytherapy rectal D2cc (EQD2) | ||||
|---|---|---|---|---|---|---|
| With spacer | Without spacer | p-value | With spacer | Without spacer | p-value | |
| Murakami et al. [22] | Median (range) 6.0 (2.3-10.3) | Median (range) 10.4 (3.9-17.0) | < 0.001 | Median (range) 53.3 (29.2-63.2) | Median (range) 64.7 (28.8-82.6) | < 0.001 |
| Kashihara et al. [17] | Median (range) 3.70 (2.56-5.76) | Median (range) 4.85 (3.23-6.65) | < 0.001 | – | – | – |
| Ahmed et al. [10] | Case 1: 1.16 Case 2: 4.99 | Case 1: 5.26 Case 2: 7.72 | – | – | – | – |
| Basu et al. [9] | 3.6 | Fraction 1: 5.7 Fraction 2: 4.8 | – | – | – | – |
| Kishi et al. [8] | 7.37 | 14.02 | – | 65.7 | 97.28 | – |
| Takatsu et al. [21] | – | – | – | Median (range) 4 fraction: 66.2 (63.9-68.9) 3 fraction: 65.4 (63.6-69.2) 2 fraction: 67.8 (62.6-69.1) | Median (range) 4 fraction: 68.8 (66.9-69.6) 3 fraction: 68.7 (66.9-69.6) 2 fraction: 68.7 (67.9-70.0) | 4 fraction: 0.01 3 fraction: < 0.01 2 fraction: 0.05 |
| Damato et al. [7] | – | – | – | Mean (standard deviation) 60 (5) | Mean (standard deviation) 74 (11) | 0.02 |
| Kobayashi et al. [23] | – | – | – | Median (range) 54.8 (39.7-71) | Median (range) 56 (38.7-68.9) | 0.272 |
Murakami et al. compared rectal dosimetric parameters in 72 patients with gynecological malignancies treated with brachytherapy using a vaginal cylinder [22]. In 15 patients with spacer compared with 57 patients without spacer, they found statistically significant reductions in the median rectal D2cc, rectal V60, and rectal V65.
Kashihara et al. assessed dosimetry on CT scans performed before and after spacer insertion in 36 patients who received gynecological brachytherapy as part of primary or salvage treatment. They found that rectum D2cc and D0.1cc were significantly reduced with spacer (p < 0.001 and p = 0.003, respectively) [17].
Ahmed et al. demonstrated that recto-vaginal spacer resulted in 35-78% and 87-95% reduction in rectum D2cc and V30, respectively, in two patients with recurrent gynecological cancers [10]. Viswanathan et al. did not report on dose volume histogram values, but stated that the addition of spacer reduced rectal doses by an average of 11% in their three patients with recurrent gynecological malignancies [16].
In their case report of a patient with stage 2B cervical cancer treated with brachytherapy, Basu et al. found a 56% relative reduction in rectum D2cc using a recto-vaginal spacer [9]. Kishi et al. described a cumulative EBRT and brachytherapy rectal D2cc of 65.7 Gy EQD2 with spacer compared with 97.28 Gy EQD2 without spacer in a patient with vaginal stump recurrence of uterine cancer [8].
Takatsu et al. carried out a planning study with 20 patients evaluating the use of gel spacer for target dose escalation and reduction of fraction number in cervical cancer brachytherapy [21]. They found significantly lower rectum D2cc values in the spacer group for virtual 4 fraction and 3 fraction brachytherapy scenarios, while achieving high high-risk clinical target volume (HR-CTV) doses. They suggested that the main limiting factor for dose escalation in the non-spacer group was rectum D2cc [21].
Damato et al. in their cadaver study showed that gel with packing decreased rectum D2cc by 22% (p = 0.02), with a 14 Gy EQD2 decrease in rectal metrics (p = 0.03) compared with packing only [7].
One study did not show a reduction in rectal doses with spacer insertion; however, the authors proposed that the spacer allowed target dose escalation whilst meeting rectal dose constraints. Kobayashi et al. analyzed 104 patients, including 52 patients with spacer and 52 patients without spacer, who underwent EBRT and brachytherapy for cervical cancer. They found no difference in rectal D2cc doses, but observed a significant increase in HR-CTV D90 in the spacer group [23].
Vesico-vaginal spacer
Four studies compared bladder dosimetry with and without vesico-vaginal spacer [7, 18, 21, 23]. One study found a statistically significant reduction in bladder dose with vesico-vaginal spacing [18].
Murakami et al. analyzed nine patients who received brachytherapy for cervical cancer with and without vesico-vaginal spacer [18]. They found that the median bladder D2cc was significantly lower with vesico-vaginal spacer vs. non-spacer (449 cGy vs. 569 cGy, p = 0.033), whilst maintaining HR-CTV coverage.
In their planning study, Takatsu et al. observed that bladder D2cc was not significantly different between the spacer and non-spacer groups for virtual 4 fraction and 3 fraction scenarios [21]. Bladder D2cc was significantly higher in the spacer group for virtual 2 fraction scenario, but still within bladder constraints. They suggested that bladder D2cc was the main limiting factor for dose escalation in the spacer group; however, they reported that the ineffectiveness of bladder spacing in their study may have been related to the spacer substance used, which was thinner compared with a previously used substance.
In a cadaver study, Damato et al. reported that a spacer with packing reduced bladder D2cc by 10%, with 12 Gy decrease in combined EBRT and brachytherapy bladder D2cc, but it was not statistically significant [7].
In a cohort of patients who received brachytherapy for cervical cancer, Kobayashi et al. suggested that spacing allowed dose escalation to the target. They demonstrated no significant difference in the median bladder D2cc between their 52 patients in the spacer group and 52 patients in the non-spacer group, but a significant increase in HR-CTV D90 in the spacer group [23].
Sigmoid and/or small bowel spacer
Four studies compared sigmoid and/or small bowel dosimetry with and without spacer between the target and the sigmoid or bowel [11, 12, 14, 16]. These studies involved injecting spacer substance through the peritoneal cavity into the desired space.
Viswanathan et al. described injection of spacer through the vaginal apex to a space between tumor and small bowel. They reported sigmoid and small bowel dose reductions of 60% and 72%, respectively, in the first patient and an average sigmoid dose reduction of 45% after spacer insertion in the two patients with sigmoid in the irradiated region [16].
Murakami et al. used “artificial ascites”, where they inserted 500 ml of normal saline percutaneously to a space between recurrent cervical tumor and sigmoid, achieving a decrease in sigmoid D0.1cc from 286 to 189 cGy per fraction [11]. Also, Karube et al. used artificial ascites, but injected 500 ml of 5% glucose with 10 ml of contrast trans-vaginally through the pouch of Douglas, and found a reduction of sigmoid D2cc dose from 229 cGy to 84 cGy as well as intestinal D2cc dose from 350 cGy to 0 cGy [12].
Additionally, Sakuramachi et al. attempted artificial ascites in a patient with cervical cancer using 500 ml of normal saline trans-vaginally through the pouch of Douglas, but this failed to provide adequate sigmoid spacing, although they did report a lower sigmoid D2cc compared with that without spacing (2.7 Gy vs. 4.3 Gy) [14]. Subsequently, they injected a hydrogel spacer into meso-sigmoid trans-vaginally and found similar sigmoid D2cc values, but they were able to escalate HR-CTV dose to at least 7 Gy for the 3rd and 4th fraction compared with no spacer (4.2 Gy) [14].
High-risk clinical target volume
Six studies reported on comparison of HR-CTV dosimetry with and without spacers [9, 14, 17, 18, 21, 23]. Studies by Kashihara et al. [17] and Takatsu et al. [21] suggested no significant difference in HR-CTV D90 doses with or without spacer. Murakami et al. reported a trend towards higher HR-CTV D90 doses with vesico-vaginal spacer (p = 0.085) [18], whereas Basu et al. [9] and Sakuramachi et al. [14] reported higher absolute HR-CTV D90 doses with fractions using spacer compared with fractions without spacer.
Kobayashi et al. found that HR-CTV D90 doses were significantly higher in the spacer group (79.4 Gy, range 52.6-97.5 Gy) compared with the non-spacer group (76 Gy, range 63.7-99.5 Gy) (p = 0.017), with no significant difference in the rectum D2cc (p = 0.272) and bladder D2cc (p = 0.628) between the groups [23]. The authors stated that they prioritized dose escalation to HR-CTV, provided that OARs doses were still within dose constraints, particularly if there was a poor tumor response.
Toxicities
Of the 17 studies, 12 did not report on acute toxicities and nine did not report on late toxicities. Three studies observed no acute toxicities [9, 13, 14], whilst five studies stated that no late toxicities were noted within follow-up periods in their reports [8-10, 13, 15]. Ahmed et al. described grade 1 acute toxicities of cystitis, proctitis, and dermatitis during EBRT as well as no adverse genitourinary (GU) or gastrointestinal (GI) symptoms at 3 months [10]. Three studies, all from the same center, reported serious late toxicities [17, 22, 23].
Kashihara et al. described no acute or late GI or GU toxicities in patients who received upfront brachytherapy, but observed severe toxicities in two patients who received salvage brachytherapy after prior radiation therapy. One patient developed grade 3 rectal bleeding and the other developed recto-vaginal and vesico-vaginal fistulas [17].
Kobayashi et al. reported grade 3+ GU late toxicity rate of 1.9% and GI late toxicity rate of 3.8% in patients with spacer insertion. They reported grade 3+ GU late toxicities in one patient in the non-spacer group (nephritis) and one patient in the spacer group (vesico-vaginal fistula). Grade 3+ GI late toxicities were seen in 5 patients in the non-spacer group (2 with rectal bleeding, 2 with recto-vaginal fistula, and 1 with ileus) and 2 patients in the spacer group (1 with recto-vaginal fistula and 1 with ileus). There were no significant differences in morbidity between the groups (GU, p = 0.65; GI, p = 0.29) [23].
In their study of patients receiving vaginal cylinder brachytherapy for gynecological cancers, Murakami et al. found that 30 patients (41.7%) experienced grade 1-2 rectal bleeding (grade 1, 28 patients; grade 2, 2 patients) and the median time to rectal bleeding from radiation starting was 30.1 months (range, 3-71.1 months). They also observed significantly less rectal bleeding in patients with spacer (2/15 patients, 13.3%) vs. no spacer (28/57 patients, 49.1%) (p = 0.01) [22]. Patients with rectal bleeding were found to have statistically significantly higher rectal D2cc compared with those without spacer (p = 0.01). According to their analysis, the estimated rectal D2cc dose that resulted in a 10% and 20% probability of greater than grade 1 rectal bleeding was 53.1 Gy and 57.7 Gy, respectively. No patients with rectal fistula, stenosis, or ulceration were observed in their study [22].
Tumor outcomes
Twelve studies did not report on tumor outcomes, and five studies discussed tumor outcomes; however, they differed in reporting parameters [8, 9, 13, 15, 23]. Kobayashi et al. reported 2-year local control rate of 85.9% in the non-spacer group vs. 91.9% in the spacer group, which was not statistically significant (p = 0.313) [23]. Marnitz et al. observed that 4 of 5 patients had no tumor cells on diagnostic curettage at least 3 months after treatment, and they were disease-free at 12 months, while one patient had residual tumor and synchronous pulmonary metastases [15]. Kishi et al. reported response in the treated vaginal stump recurrence with no FDG uptake on PET at 6, 12, 18, 24, and 30 months after treatment [8]. However, the patient developed low vaginal recurrence one year after treatment, and was salvaged with EBRT and brachytherapy. She was alive, but experienced lung metastases at 3 years. Takagawa et al. described a tumor size reduction on MRIs up to 8 months post-brachytherapy; however, the patient died of systemic progression 21 months after interstitial brachytherapy [13]. Basu et al. presented a patient who was alive with no evidence of a recurrence, although follow-up period was not specified [9].
Discussion
In this review, 17 studies were identified, which evaluated the use of injectable bio-absorbable spacers in gynecological cancer brachytherapy with various endpoints.
Material
There was a range of spacer materials used in the reported literature, with differing properties relating to reabsorption times, pre-mixing requirements, radio-opaqueness, and time constraints for injection. However, the studies were not consistent in their reporting of product properties, making direct comparison challenging.
Spacer reabsorption ranged from 1-4 hours [8] to 6 months [13], depending on the base product, concentration, and molecular weight [8, 19]. The rapid reabsorption seen with sodium hyaluronate products, Suvenyl®, and MucoUp® was felt to be advantageous in brachytherapy in the event of injection into the incorrect anatomical location [19], avoiding potential complications with enduring inappropriately placed spacer. However, re-insertion of spacer with each brachytherapy fraction may increase the risk of complications due to repeated invasive procedures compared with single-insertion of a spacer with a longer half-life.
Some products, such as the polyethylene glycol hydrogel, SpaceOARTM, require pre-mixing of a powder and dilutant compared with the simpler approach of an already mixed single-syringe solution, such as TraceIT [10]. TraceIT, an iodinated hydrogel, is radio-opaque spacer with multimodal visibility [7], while products, such as Suvenyl®, MucoUp®, saline, and 5% glucose, require mixing with contrast for visibility on CT [8, 12, 14, 17-22]. Time constraints during injection due to polymerization were noted in various products, e.g., Space-OARTM [10], whilst others were more structurally stable.
Timing of spacer insertion
The insertion of spacer predominantly occurred at the time of brachytherapy. Only one paper described a patient, in whom spacer insertion occurred prior to EBRT and brachytherapy [10]. Availability of a theatre space, appropriately trained personnel, equipment, and time, may be potential barriers for insertion of spacers prior to EBRT in patients receiving both EBRT and brachytherapy for gynecological cancers. Since anesthesia is already administered for a brachytherapy applicator insertion, the spacer may be inserted at the same time, which eliminates the need for an additional theatre session, anesthesia, and related costs. Furthermore, optimal care pathways dictating time frames to commencement of treatment from decision to treat [24] as well as patient symptoms, such as bleeding, may necessitate more urgent commencement of EBRT, thereby precluding sufficient time for spacer insertion pre-EBRT. Finally, there may be concern about microscopic disease in the recto-vaginal and vesico-vaginal spaces, and therefore inserting a spacer with a short half-life post-EBRT allows adequate dose to the microscopic disease, while facilitating reduced dose to OARs during brachytherapy [25].
Technique
The majority of insertions were delivered in the lithotomy position under TRUS guidance with a trans-vaginal approach. However, Kishi et al. suggested that para-perineal insertion offers advantages over trans-vaginal insertion, such as a wider range of needle angles and positional stability [8]. Most spacers were inserted prior to applicator insertion, although Murakami et al. stated that timing of spacer insertion to tandem insertion was insignificant [22]. The authors recommended spacer insertion prior to interstitial catheter insertion, as interstitial needles may interfere with TRUS image quality [22].
Spacers were more commonly inserted in the recto-vaginal septum compared with the vesico-vaginal septum. The vesico-vaginal space is thinner, and therefore insertion of spacer is more difficult with higher rates of failure, especially at the beginning of a learning process [20]. Additionally, the recommended dose constraints for the rectum are stricter than that for the bladder, and therefore, rectal sparing is potentially of greater priority.
There were few reports examining spacing of the small bowel or sigmoid colon [11, 12, 14, 16]. The infrequent use may be related to additional risks involved with intra-peritoneal spacing, such as peritoneal dissemination, iatrogenic tumor implantation, and bowel injury [12, 14]. Moreover, satisfactory spacing may not be achievable due to dissipation of the spacer into the peritoneal cavity [19].
Dosimetric impact
The impact of spacer use on dosimetry varied amongst the studies depending on the clinical priority. In scenarios where OARs constraints limited ability to achieve sufficient dose to the target, the spacer allowed HR-CTV dose escalation while limiting OARs dose to within constraints [14, 23]. Alternatively, if adequate dose was delivered to HR-CTV without spacer, the spacer created a distance and facilitated further reduction in OARs dose [17, 18, 21].
Complications
Spacer insertion seemed to be well-tolerated, with no significant procedure-related complications described. However, the investigated patient samples were small, and there was inconsistent reporting within the studies.
Limitations
A major limitation of this review is the low certainty evidence consisting of case reports, small case series, retrospective cohort studies, and dosimetric studies. In addition, eight of the 17 studies originated from the same center, with marginally differing inclusion criteria and aims [11, 12, 14, 17, 18, 20, 22, 23]. This raises the possibility of overlapping patient populations, which introduces bias and limits the generalizability of findings. However, given the sparse data available on this topic and the extensive experience of the center, their studies provide important insights.
Although all the studies provide valuable information on spacer use in gynecological cancer brachytherapy, there is limited evidence on suitable patient selection for spacer utilization, technical aspects of spacer insertion, potential complications, spacer stability, oncological outcomes, late toxicities, patient-reported outcomes, and cost-effectiveness. Given the relatively lower incidence of gynecological malignancies compared with other cancers, such prostate cancer, where spacers are most commonly used, it may be challenging to conduct a randomized controlled study. However, prospective studies using spacers in gynecological cancer brachytherapy would allow better assessment of utility.
Conclusions
The use of injectable bio-absorbable spacers is a promising approach for improving the therapeutic ratio in gynecological cancer brachytherapy. Prospective studies are required to examine suitable patient cohorts, optimal technique, spacer stability, oncological outcomes, late toxicities, patient-reported outcomes, and cost-effectiveness.