Purpose
Ocular treatment aiming at preserving an eye with a useful vision is now a standard of care in current management of uveal melanoma. Most patients are treated with radiotherapy, and the radiation is delivered by the means of episcleral brachytherapy plaques, proton beam, or stereotactic radiotherapy (SRT). Plaque radiotherapy combined with transpupillary thermotherapy can provide intraocular tumor control, with local tumor recurrence of only 3% at 5-year follow-up [1]. However, potential ocular complications of plaque brachytherapy treatment include radiation reti-nopathy (RR) or radiation maculopathy (RM), radiation-induced cataract, radiation neuropathy, secondary glaucoma, vitreous hemorrhage, retinal detachment or even scleral necrosis, strabismus and toxic tumor syndrome [2, 3].
Unfortunately, radiation also damages healthy chorioretinal tissue, leading to maculopathy and/or optic disc edema that may compromise patients’ vision years after successful local treatment. Radiation maculopathy clinically can be observed as chronic macular edema (ME). Until now, several preventive strategies have been developed; however, the effective RM treatment still remains challenging.
This review summarizes the current understanding of potential radiation damage to the ocular tissues, clinical features of RR, and recent developments in ophthalmic multimodal imaging techniques. Highlights the newest treatment strategies and some promising prophylactic options used by ophthalmologists in the management of RM after episcleral brachytherapy (BT).
Radiation retinopathy is a chronic, progressive vasculopathy that develops after exposure to any type of radiation. It is a broad term that refers to all retinal vascular changes caused by radiation, and includes both non-proliferative and proliferative retinopathy and/or macular edema. Most frequently, it occurs after a treatment of intraocular tumors (e.g., uveal melanomas), but also after treatment of other tumors of the head and neck region (i.e., nasopharynx, sinuses, and central nervous system) [4]. The radiation damage leads to chronic ischemia in the irradiated area, which results in vascular changes, such as those observed in diabetic retinopathy. Clinically, it would manifest with retinal microaneurysms, telangie-ctasias, hard exudates, cotton wool spots, and retinal hemorrhages, as shown in Figure 1. Chronic retinal ischemia may lead to neovascularization of the optic nerve disc (NVD) or retina (NVR), which can contribute to vision-threatening complications, such as vitreous hemorrhage and neovascular glaucoma. These changes may be accompanied by choroidal vessels damage (choroidopathy) or atrophy of the retinal pigment epithelium (RPE) [2, 5].
Fig. 1
Radiation retinopathy following ruthenium-106 plaque brachytherapy in two patients: color (A, C) and autofluorescence imaging (B, D) of the retinal fundus (Zeiss Clarus 500 Fundus Camera)
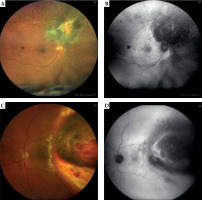
Epidemiology
Radiation retinopathy can start several months to several years after ocular treatment, affecting about 20-53% of patients after BT, depending on the location of tumor and radionuclide used [6, 7]. Radiation macu-lopathy was reported as the most common radiation complication after ruthenium BT, occurring in 24.2% of patients [8]. After SRT, RR occurred in 27.7-42% (Cyber Knife) to 34.5% (Gamma Knife) of patients, depending on a study, with a median onset of 13.7 months [9-11]. The RR risk following proton beam irradiation has been reported even more frequently, ranging to 85-90%, but proton beam-irradiated tumors normally are located more posteriorly [12].
Etiology
Ionizing radiation acts directly by disrupting chemical bonds in molecules and damaging DNA in dividing cells. It also indirectly interacts with organic matter by producing free radicals that cause extensive damage to cellular structures, and are cytotoxic. Both mechanisms result in damaging tumor cells, but also surrounding tissues.
Vascular endothelial cells are particularly susceptible to radiation damage affecting cell loss and vascular wall weakening [16]. Over time, the damaged capillaries occlude and provide retinal ischemia and neovasculari-zation. Moreover, histopathology studies on irradiated lesions from enucleated eyes after BT have demonstrated less mitotic activity and more inflammation and macrophages infiltration compared with primary tumor tissue [13]. One of the hypotheses emphasizes the importance of inflammatory component in the development of radia-tion damage, which may be observed as small, hyperreflective retinal foci on OCT scans. These may represent aggregates of activated microglial cells [14, 15].
The irradiated RPE may lose melanin, accumulate lipofuscin, or develop atrophy or hyperplasia [16].
More recently, Platt et al. investigated 26 eyes that had undergone plaque BT because of uveal melanoma prior to enucleation. The authors conducted a histopathologic analysis of all 26 eyes with special emphasis on the choroidal changes. Out of these 26 eyes, 18 demonstrated evidence of radiation-induced vasculopathy, 55% had RR, and 89% had radiation choroidal vasculopathy [17].
Risk factors
Risk factors of developing BT-induced RR include total radiation dose, tumor thickness (more than 4 mm), and tumor location next to functionally important structures, such as fovea and optic disc [18].
With regards to teletherapy used for uveal melanoma treatment, according to some authors, the size of tumor base, tumor thickness, and location of tumor have predictive values for RR, and eyes with larger basal diameter are more likely to develop RR after gamma knife radiosurgery [19]. Other authors report that RR is not significantly associated with the mean radiation dose to the tumor, tumor thickness or tumor its distance from the fovea [20]. Co-existing diabetes and increased risk of RR are supported only by case series reports [21, 22].
Shields et al. reported poor visual outcome (≤ 20/200) at 5 years in 30% of patients with medium-sized melanoma, and 64% with large melanoma [23]. In the Collaborative Ocular Melanoma Study (COMS), patients were followed for 3 years after brachytherapy and 43% had a final visual acuity (VA) of 20/200 or less [24]. Recently published investigations presented new normograms that could predict the visual outcome post radiation treatment, and included the use of post-operative anti-VEGF agents as a standard of care [25, 26].
Imaging
Multimodal imaging is crucial in proper diagnosis and timely implementation of treatment. Presymptoma-tic detection of RM allows for identifying the onset of RR on time. Such a complex imaging approach has been applied to several retinal diseases so far and it’s still developing in RR. Depending on the type of imaging used, different classification of RM or RR were proposed, as shown in detail in Table 1.
Table 1
Classification of radiation retinopathy (RR) based on ophthalmic multi-modal imaging
| Method of imaging | Classification of radiation retinopathy/maculopathy |
|---|---|
| Ophthalmoscopic findings and fluorescein angiography [36] | Stage 1: Extramacular ischemic changes Stage 2: Macular ischemic changes Stage 3: Additional macular edema and extramacular retinal neovascularization Stage 4: Vitreous hemorrhage Stage 5: Disc areas of retinal ischemia macular or extramacular |
| Fluorescein angiography [27] | Non-ischemic and ischemic radiation maculopathy Focal, diffuse, and mixed radiation macular edema |
| Widefield fluorescein angiography [74] | Grade 0: Normal Grade 1: Late foveal leakage Grade 2: Late peripheral leakage Grade 3: Presence of non-perfusion Grade 4: Retinal neovascularization |
| OCT examination [5] | Grade 1: Extrafoveolar non-cystoid edema Grade 2: Extrafoveolar cystoid edema Grade 3: Foveolar non-cystoid edema Grade 4: Mild-to-moderate foveolar cystoid edema Grade 5: Severe foveolar cystoid edema |
| Angiography of OCT, SD-OCT, and ophthalmoscopic findings [75] | Grade 0: –,–,–,– Grade 1: +,–,–,– Grade 2: +,+,–,– Grade 3: +,+,+,– Grade 4: ++,+,+,+ Grade 5: Unreadable,++,++,+ Clinical features detected in OCTA, OCT thickness and cysts, ophthalmoscopic features |
| Multi-modal imaging and visual acuity-oriented classification of RM [35] | Cx: Vertical size of the largest macular cyst cannot be assessed C0: No evidence of measurable cysts Cn: n indicate the vertical size of the largest macular cyst in µm Jx: Presence of IS/OS junction alterations cannot be assessed J0: No evidence of IS/OS junction alterations J1: Presence of IS/OS junction alterations Ax: Presence of RPE atrophy cannot be assessed A0: No evidence of RPE atrophy A1: Presence of RPE atrophy Cyst junction atrophy (CJA) classification: vertical thickness of the largest macular cyst (C parameter), IS/OS layer disruption (J parameter), and presence of foveal RPE atrophy (A parameter) |
Table 2
Anti-VEGF treatment in radiation retinopathy. Comparison of studies’ design and results
| Anty-VEGF intra- vitreal injection | Study design/(n of patients) | Medication dose | Scheme of administration | Follow-up period | Comments on results |
|---|---|---|---|---|---|
| Aflibercept [61] | Randomized, clinical prospective study Evaluation of 2 treatment approaches/(20)/(20) | 2 mg/0.05 ml | Every 6 weeks (9 injections) | 60 weeks | Baseline study entry mean BCVA was 20/63 and was maintained at 20/62 at study conclusion of 60 weeks. Almost half of all treated patients maintained BCVA 20/50 or better. Mean CRF SD-OCT was significantly decreased from 432 µm to 294 µm. No difference was found between the two groups |
| 2 mg/0.05 ml | Treat and extend (mean, 8.4 injections) | 60 weeks | |||
| Aflibercept [62] | Prospective, interventional case series report/(9 eyes) | 2 mg/0.05 ml | PRN (mean of 4.4 injections were given) | 24 months | Functional (BCVA) and anatomical (CRT SD-OCT) improvement. At the end of follow-up, mean BCVA was significantly improved, from 0.9 logMAR at baseline to 0.56 logMAR |
| Bevacizumab [51] | (6) | 1.25 mg/0.05 ml | PRN (every 6-8 weeks) | Mean, 4.7 months (range, 2-8 months) | 2 patients resulted in improvement of BCVA and 4 had it stable |
| Bevacizumab [53] | (21) | 1.25 mg/0.05 ml | PRN (mean of 3.8 injections) | Mean, 7.8 months (range, 2-18 months) | 18 patients (86) had stable or improved BCVA |
| Bevacizumab [52] | Retrospective study/(159) | 1.25 mg/0.05 ml | PRN (81 patients were given a mean of 5 injections) | 3 years | 81 patients (50.9%) demonstrated BCVA 20/50 or better. Mean BCVA prior to plaque placement was 20/34. At the time of first anti-VEGF injection, mean BCVA was 20/43, which improved to 20/31 at 34.6 months after brachytherapy |
| Bevacizumab [54] | Retrospective review/(31) | 1.25 mg | Monthly 3×, then PRN (average, 5 injections) | Average of 19 months (range, 0-43 months) | Average BCVA on the day of first intra-vitreal injection was 0.7 logMAR and decreased to 1.3 logMAR at last follow-up. After initiating injection therapy, the mean visual acuity remained stable for 9 months. Patients benefited most from injections administered every 90 days or sooner |
| Bevacizumab [55] | Non-comparative, interventional case series/(36) | 1.5 mg/0.06 ml | Every 4 months | 4-6 months | Improvement of BCVA in 42% of the treated eyes, stable in 42% of eyes, and decline in macular edema in 56% of patients. There was a decrease in mean CFT from 482 µm before injection to 284 µm at 6 weeks, but this increased to 449 µm at 4 months after the injection |
| Bevacizumab [56] | For recalcitrant to 1.25 mg bevacizumab/(15) | 2.5 mg/0.5 ml | Every 3 months | 9 months | Mean BCVA improved from 0.55 logMAR to 0.48 logMAR, and mean CMT reduced significantly from 406 µm to 360 µm after 3 months of follow-up. At final follow-up at 9 months, CMT was 395 µm and BCVA was 0.51 logMAR. Response with significant CMT reduction, but not in BCVA |
| Ranibizumab [57] | Phase 1, open-label, single-center clinical trial/(5) | 0.5 mg | Monthly 4×, then PRN (mean of 8.2 injection) | 8 months | BCVA improved in 4 of 5 patients (80%) by a mean of 9.5 letters. Mean initial CFT was 416 µm, mean final CFT was 270 µm (reduction in all cases) |
| Ranibizumab [58] | Prospective, randomized clinical trial/(8)/(16)/(16) | 0.5 mg | Monthly | 12 months | There was a significant difference in mean BCVA at 1 year among all 3 cohorts, with the most significant gains in the monthly group. Mean CMT decreased in all 3 cohorts |
| 0.5 mg | Monthly and TRP | 12 months | |||
| 0.5 mg | Monthly 3×, then PRN and TRP | 12 months | |||
| Ranibizumab [60] | Prospective randomized controlled trial. Comparison of intravitreal ranibizumab versus laser photocoagulation/(15)/(16) | 0.5 mg | Monthly 2×, then PRN (median 5 injections) | 12 months | Significant improvement in BCVA from baseline: 0.16 logMAR to 0.03 logMAR for ranibizumab at 26 weeks end-point. The effect disappeared between two groups at week 52 |
| Laser therapy | Focal laser treatment of the macula or peripheral laser treatment of the ischemic retina | 12 months | |||
| Ranibizumab [59] | Non-randomized prospective clinical trial. For patients with recalcitrant radiation retinopathy who were failing 0.5 mg ranibizumab or 1.25 mg bevacizumab therapy/(5)/(5) | 2 mg | Every 30 days ±7days for 12 months (mean, 10.2 injections) | 12 months | Mean change in BCVA was +3.3 letters at 6 months and +0.7 letters at 1 year. Initial mean CFT was 428 µm and decreased to 333 µm (80% of patients demonstrated a statistically significant reduction in CFT) |
| 2 mg | Every 30 days ±7days for 4 months, then every month (mean, 10.8 injections) | 12 months |
Fluorescein angiography (FA) shows microvascular features of RR. Classification of RR with combination of ophthalmoscopic and FA findings includes the identification of non-ischemic and ischemic RM, focal, diffuse, and mixed radiation-induced macular edema (ME) [27].
Indocyanine green angiography detects associated damage of choroidal vasculature (atrophy or remodeling of vessels). Early changes can indicate peritumoral atrophy of RPE and irregular closure of choriocapillaris, arterioles and venules [28], which are particularly useful in guiding retinal photocoagulation.
Enhanced depth imaging optical coherence tomography (EDI-OCT) allows for further investigation of post-irradiation choroidal damage [28]. OCT-based grading scheme is ideal for early identification of radiation-induced ME, and helps in assessing the severity of maculopathy. Horgan et al. proposed a five-point OCT-based grading scale for RM, depending on localization of intraretinal cysts, as shown in Table 1 [5, 29]. Patients with early or mild RR may be asymptomatic while some changes in OCT or optical coherence tomography angiography (OCTA) are clearly visible. In their study on 135 patients after iodine (125I) brachytherapy, ME occurred on average 12 months after radiotherapy, but at the earliest even after 4 months. 33% of patients did not have symptoms of RR, so the ME visible in OCT preceded clinical symptoms of RR by 5 months, and made the OCT an important tool in diagnosis and monitoring [29].
OCT-A is very sensitive in the detection of the earliest manifestations of RM showing parafoveal capillary network abnormalities. The enlargement of foveal avascular zone (FAZ) and decreased parafoveal capillary density of both superficial and deep capillary plexus in eyes after brachytherapy of choroidal melanoma with no clinical evidence of RR were demonstrated [30-33].
Finally, Parrozani and Midena proposed a classification of RM based on features detectable with multimodal imaging, as shown in Table 1. According to the authors, some parameters as macular cysts are biomarkers related to VA [34, 35]. The OCT and OCT-A symptoms of RR are discussed in Figure 2.
Fig. 2
Right eye of the 47-year-old male patient sixteen months after ruthenium plaque brachytherapy: color (A) and auto-fluorescence (B) images of the fundus. The images present macular edema with intra-retinal cysts visible in OCT scan (C) and parafoveal vessels droplets in OCTA scans of the macula (retinal capillary network: D) superficial capillary plexus, E) deep capillary plexus, F) density map of the vessels). Scans were captured by DRI OCT Triton, Topcon
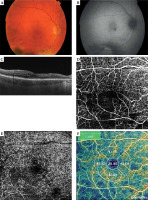
Treatment
Treatment of RR remains challenging due to the lack of large randomized clinical trials and clear clinical guidelines. Treatment options comprise laser photocoagulation, intravitreal steroids, intravitreal anti-vascular endothelial growth factors (anti-VEGFs), and surgery. The main goal of the treatment is to improve visual function and/or prevent further visual loss. Although there are constant improvements in diagnostic tools and treatment options for RR, a considerable number of eyes, particularly those with proliferative RR or further complications, still loose vision.
Laser therapy
Several studies demonstrated argon laser (focal or grid) efficacy in the reduction of macular thickness, but they were usually only short-term improvements, allowing for the maintenance of VA in a small group of patients [36, 37]. In a group of 45 eyes, Finger et al. demonstrated that sector argon laser photocoagulation resulted in regression of clinically evident RR in 64.4% patients and 15.5% of them lost more than 3 lines in VA due to MR [36].
Hykin et al. demonstrated that focal laser therapy in 42% of patients (8 eyes) had improved VA comparing with observed controls at 6 months follow-up, but the two groups did not differ after 24 months [37]. For the treatment of proliferative RR, peripheral laser photocoagulation is considered, especially in case of retinal ische-mia in extramacular localization or neovascularization. Bianciotto et al. found that panretinal photocoagulation causes regression of neovascularization in 66% of eyes with proliferative RR [38]. With regrads to micropulse laser therapy (MPLT), only case reports are available with short follow-up, resulting in improving symptoms and anatomical benefits [39, 40].
Glucocorticosteroids
Intravitreal steroids are widely used in the treatment of ME, and therefore can be also employed in the treatment of RM. Some studies suggest that intravitreal triamcinolone acetonide (4 mg/0.1 ml) (TA) temporarily reduces ME and improves vision. Shields et al. reported that single intra-vitreal injection of 4 mg TA improved or stabilized VA in 91% eyes at 1 month, and in 45% eyes at 6 months after uveal melanoma BT [41].
Steroids are believed to restore the integrity of inner retinal barrier by increasing tight junction protein and upregulating adenosine; therefore, they may complement the action of anti-VEGF medications in the event of incomplete response to current treatment [42, 43]. They also reduce the inflammatory response that appears during RR with fluid leakage and lipid exudation. Some studies report 4 mg TA as an adjuvant therapy to bevacizumab in incomplete response to treatment or co-therapy in radiation-induced ME [44, 45].
Various authors investigated the efficacy of 0.7 mg intravitreal dexamethasone (DEX) implant or off-label use of fluocinolone acetonide (FA) slow-release implants [46-49]. An improvement of VA has been reported with the use of DEX implant, but with no statistical significance [46, 47]. Of note, the efficacy of intravitreal slow-release implant of 0.19 mg fluocinolone acetonide (FA) was demonstrated in a small group of patients, allowing for the stabilization of VA at 8 months follow-up [49].
Anti-vascular endothelial growth factor (anti-vegf) therapy
Anti-VEGF therapy is a common treatment used nowadays in ophthalmology to manage various retinal diseases. Bevacizumab is the whole anti-VEGF antibody, while ranibizumab is a recombinant humanized IgG1 monoclonal antibody fragment. Both bind to all isoforms of vascular endothelial growth factor A (VEGF-A), but ranibizumab is believed to penetrate through the retinal tissue better and has higher affinity to VEGF-A than bevacizumab. Aflibercept is a fusion protein made up of parts of extracellular domain of human VEGF receptors fused with a part of human immunoglobulin. It binds to all isoforms of VEGF-A, and also to vascular endothe-lial growth factor B (VEGF-B) and placental growth factor (PGF) [50]. The VEGFs increase vascular permeability and stimulate the division of endothelial cells and formation of new blood vessels (neovascularization process). Anti-VEGF treatment inhibits both processes resulting in reduction of ME and improvement of visual function. However, to achieve these benefits, multiple injections over a certain period of time are required. The anti-VEGF treatment can be administered in fixed time intervals (i.e., every 3 to 4 months) or adjusted as needed (i.e., 3 injections every 3 weeks) and then as needed (pro-re-nata – PRN) to obtain the resolution of ME. Of note, some studies have also found variable responses to treatment according to different treatment protocols [51-54].
Bevacizumab
Most of the trials on RR treatment concerned bevacizumab as the most low-cost option (which is used off-label for intravitreal injections), but there were often small groups of patients, case series, or retrospective trials.
Intravitreal injection of 1.25 mg bevacizumab (i.e., PRN) caused regression of ME with stabilization or improvement of VA in most patients [51-53], or in the form of loading phase of 3 injections and then administered PRN [54], or injections every 4 months [55]. Higher doses of bevacizumab (2.5 mg) were administered in recalcitrant ME [56]. In the protocol using intravitreal bevacizumab injection every 3 months in a group of 15 patients, there were no benefits during 9-month follow-up in anatomical improvement of the macula or VA [56].
Ranibizumab
Ranibizumab was investigated in different doses (0.5 or 2 mg) as a first-line treatment in RM or in recalcitrant ME, and in comparison with other treatment options [57-60]. Schefler et al. in their prospective trial investigated the effectiveness of three different ranibizumab treatment protocols in RR: monthly ranibizumab, monthly ranibizumab and targeted retinal photocoagulation (TRP) to areas of peripheral retinal ischemia after 1 week, or three consecutive loading doses of monthly ranibizumab, and then only treated as needed (PRN) with TRP after 1 week after first injection. They showed that ranibizu-mab improved vision and central macular thickness (CMT), and prevented visual loss for one year in every arm of the study. Moreover, monthly injections were more effective than PRN regimen, and the addition of TRP showed no benefits [58].
Finger et al. observed that intra-vitreal injections of high-dose ranibizumab (2 mg) induced significant reductions in ME in 80% cases, and maintained or improved best corrected visual acuity (BCVA) in 70% of patients who did not improve with standard dose anti-VEGF therapy. However, the studied group comprised only 10 patients with 1 year follow-up [59]. Seibel et al. in a prospective randomized controlled trial investigated the effect of intravitreal 0.5 mg ranibizumab injections versus laser photocoagulation (Radi Ret study). In 6 months follow-up, ranibizu-mab was superior to laser treatment with regards to visual function, but the positive effect disappeared if treatment was discontinued after 12 months [60].
Aflibercept
A randomized study with 2 mg aflibercept administered every 6 weeks or in a treat-and-extend approach, showed VA stabilization and ME reduction. Only 5% of eyes had a BCVA worse than 20/200. Functional and anatomic improvements were demonstrated at 12 months in both groups, with no difference in BCVA by protocol [61]. Similarly, Fallico et al. found significant improvement in VA and CMT in MR using 2 mg intravitreal aflibercept with PRN protocol, but only 9 eyes were treated [61, 62].
Next generation anti-vegf drugs
There are some drugs that are promising for the treatment of ME, including brolucizumab, faricimab, and conbercept, or technologies with a port-delivery system, but they still need investigations in RR [63]. Brolucizumab works by inhibiting the binding of VEGF-A to its receptors. Unlike full-size antibodies, this drug has a small molecular size and lacks a crystallizable fragment that allows for better tissue penetration [64, 65]. Faricimab has a dual mechanism of blocking angiopoietin-2 and VEGF-A simultaneously, so it can be an interesting treatment option for radiation-induced retinopathy with its inflammatory background [63]. Conbercept is a recombinant fusion protein that binds specifically to VEGF-B, PGF and various forms of VEGF-A [63].
Surgical treatment
Proliferative RR has been reported to develop in 3% to 25% of eyes treated with BT [7, 35]. Advanced proliferative RR complicated by vitreous hemorrhage or retinal detachment may require pars plana vitrectomy. Neovascular glaucoma remains a difficult complication that can lead to enucleation. In patients who received 125I plaque BT, secondary enucleation due to neovascular glaucoma occurred in 1-12% of treated eyes [66]. In cases of toxic tumor syndrome, surgical removal (endo-/exo-resection) of the remaining irradiated tumor tissue can be another way of treatment.
Prevention of radiation retinopathy and macular edema
In prevention of developing RR or ME, few different strategies were investigated; but mostly only on small group of patients. When qualifying patients for prophylactic treatment in these cases, the sum of benefits, including VA, must overweight the risk of complications, such as cataract, glaucoma, infections, etc. There are few treatments that patients can benefit most, including sub-Tenon’s steroids, laser, and prophylactic anti-VEGFs.
Steroids
Sub-Tenon’s triamcinolone every 4 months can be employed as prophylactic treatment. In a prospective, randomized, controlled clinical trial, periocular injection of triamcinolone acetonide (40 mg/1 ml) was administered at the time of plaque radiotherapy, and then 4 and 8 months later. ME occurred less often with statistical significance in the triamcinolone group compared with the control group up to 18 months after plaque BT (143 patients were included). At 18-month follow-up, visual loss of 3 lines or more and BCVA 5/200 on Snellen chart, occurred significantly less frequently in the TA group than in the control group (31% vs. 48%, and 5% vs. 15%, respectively). Rates of elevated intraocular pressure and cataract progression were similar in both groups [67].
Laser therapy
Laser ablation of ischemic peripheral retina in some studies were proved to reduce the incidence of retinal neo-vascular complications. Finger et al. used prophylactic sector-laser photocoagulation, and reported that 62% of patients were stable, 19% improved their BCVA at a mean follow-up of 16.5 months, but it was a small series report of 16 patients [36].
Anti-vegf drugs
As a prophylactic strategy to prevent radiation-induced ME and RR, intravitreal injections of bevacizu-mab every 4 months after plaque placement seem to be the most promising approach [68, 69].
Shah et al. in their research showed a lower percentage of eyes with ME as well as decreased BCVA compared with the control group. The bevacizumab group of 292 patients received intra-vitreal 1.5 mg bevacizumab injection at the time of plaque removal, and 6 subsequent injections every 4 months over 2 years. This group demonstrated less frequently OCT-evident ME (26% vs. 40% of the control group), clinically evident RM (16% vs. 31%), loss of 3 lines or more on Snellen BCVA (33% vs. 57%), and poor visual acuity (BCVA worse than 5/200 on Snellen chart, 15% vs. 28%) over a period of 2 years compared with the non-treatment group [68].
Shields et al. in a retrospective, non-randomized research administered 1.25 mg bevacizumab to 1,131 eyes with irradiated uveal melanoma (bevacizumab group) and compared their results with 117 eyes without bevacizumab after BT. Treatment protocol included intravitreal injections with 4-month intervals over 2 years following brachytherapy. The bevacizumab group demonstrated less OCT-evident ME and fewer clinical signs of RM at 24-, 36-, and 48-month follow-up, and statistically significant better VA outcomes at all time points (0.54 log MAR vs. 2.00 log MAR at 48 months’ time point). There was no ocular or systemic adverse bevacizumab reaction in any patient [69].
Comparable results in smaller groups of patients were achieved by Powell et al. They analyzed a group of 14 patients, to whom bevacizumab was administered every 4-6 weeks over 6 months after BT. It prevented or delayed the onset of RM and VA loss [70]. These patients were compared with the control group (14 historical patients diagnosed with choroidal melanoma and treated with palladium-103 plaque BT), case-matched by radiation dose to fovea, proximity to fovea, and size of tumors. When compared with their VA measured at the time of diagnosis, 64.3% in the anti-VEGF-treated group showed improvement or no change in VA, in contrast to 28.6% in the case-matched group. No patient in the anti-VEGF group lost more than 3 lines of vision compared with 10 patients (71.4%) in the case-matched group. At last follow-up, 50% patients demonstrated OCT-detected RM, as compared with 85.7% in the control group. However, this was non-randomized study with a retrospective design and a small number of patients [70].
Another approach propose the use of optimized radiation dosage and delivery techniques that can help reduce the risk of RR and ME. This includes apex dose reduction, eccentric plaques or displacement of radioactive seeds [70-72]. However, greater doses to tumor apex result in better local control of disease [73]. Early detection and treatment of radiation damage can help prevent or minimize visual loss. Therefore, patients who undergo BT should have regular follow-ups.
Conclusions
Radiation chorioretinopathy is a vision-threatening condition. The current management of radiation maculopathy and retinopathy is based mostly on anti-VEGF agents, laser therapy and steroids. The final choice of the treatment strategy depends on the severity of radiation damage; however, in the majority of ME cases, intravitreal anti-VEGFs would be the first choice. Further randomized clinical trials with larger cohorts of patients and longer follow-ups are needed to establish an effective protocol of treatment as well as prevention of radiation maculopathy following episcleral plaque placement.



