Purpose
Non-melanoma skin cancer (NMSC) represents the most widespread form of cancer globally, with a significant upward trend in its incidence. In 2019, 6.35 million new cases of NMSC were reported, causing 56,054 deaths and producing 1.18 million disability-adjusted life years (DALYs) [1]. The head and neck region, a cosmetically sensitive and functionally critical area, bears a significant burden of this cancer [2].
Surgical excision, particularly Moh’s microsurgery, remains the gold standard for treating NMSC, but it presents significant challenges in the head and neck region. Tumors’ adjacent vital structures, such as the eyes, ears, or nose, require a delicate balance between achieving clear margins and preserving functionality and esthetic impairments. Additionally, elderly patients or those with significant medical comorbidities may not be ideal candidates for surgery. For these high-risk surgical candidates, radiotherapy provides a valuable alternative, offering effective treatment while addressing these complex considerations [3-6].
There are three main techniques regarding the application of radiotherapy for NMSC. External beam radiotherapy (EBRT) utilizes external radiation photon beams to deliver radiation to the tumor [7]. Electron beam radiotherapy uses electron beams to deliver radiation, offering improved tissue sparing and cosmetic results compared with traditional large fraction superficial X-rays [8]. The application of high-dose-rate brachytherapy (HDR-BT) for treating NMSC has been in clinical practice for a while. In 1999, Köhler-Brock et al. documented a 91% complete remission rate, with a cumulative dosage ranging from 30 to 40 Gy, indicating this method’s effectiveness in treating surface lesions [9].
The application of HDR-BT using both custom molds or conical-shaped applicators, such as Leipzig surface applicators, offers high local control rates and excellent cosmetic outcomes, making it a compelling alternative to surgery for NMSC treatment, especially for lesions on the face [10, 11]. HDR surface mold brachytherapy is a targeted approach that involves crafting a customized mold from a pliable material that precisely conforms to the treatment area’s contours. Radioactive sources are then meticulously embedded within this mold, allowing for high-dose delivery in a localized area and minimizing exposure to healthy tissues. They provide more customization and are suitable for complex anatomical areas, achieving local control rates of up to 100% [12, 13]. Moreover, studies have shown that CT-based surface mold brachytherapy provides better conformity for small, irregular surfaces (e.g., the nose) than electrons, with excellent dose coverage and slightly higher skin doses [14]. Leipzig surface applicators, while less customizable, still offer notable local control rates, making them practical for more straightforward treatment areas [9, 10, 15, 16].
Different sources, including iridium-192 (192Ir), cobalt-60 (60Co), and ytterbium-169 (169Yb), have been studied with conical applicators for treating NMSC. Iridium-192 is the most widely utilized radiation source in HDR-BT, since it possesses favorable physical properties, and is beneficial for treating superficial lesions with rapid dose fall-off. It is ideal for challenging locations, such as the nose and earlobe. 60Co, with a longer half-life than the ytterbium-169 (169Yb) and 192Ir (5.26 years, 32.02 days, and 73.83, respectively), reduces the source exchange frequency while maintaining comparable dose distributions. However, due to the higher average energy (1.25 MeV) than 169Yb (92.7 KeV) and 192Ir (380 KeV), more shielding is required to reduce radiation exposure to normal tissues. The results suggested that the 60Co source with double-wall applicator or 169Yb with standard applicator could be viable alternatives for treating skin cancers instead of the 192Ir source with conical standard applicator [14, 17]. The comparative efficacy and suitability of different sources in mold brachytherapy applications should also be noted, as they provide tailored treatment options based on specific clinical scenarios.
Good practice of surface mold brachytherapy requires considering several aspects of the procedure. These include patient selection by comparison with other radiotherapy techniques and thickness of the lesion or tumor bed, mold design and fabrication, radioactive source selection and placement, treatment planning and dosimetry, treatment delivery, and monitoring. This retrospective analysis aimed to contribute valuable insights into its role as a safe and effective treatment option for head and neck NMSC by meticulously examining these aspects of surface mold brachytherapy techniques. We have reported the outcomes of our institution for surface mold brachytherapy using our previous 192Ir source [18], but we intended to report our results with more specific technical details using our HDR brachytherapy afterloader machine that uses a 60Co source.
Material and methods
Patient characteristics
This retrospective cohort study included patients with histologically confirmed NMSC, who sought treatment at the Cancer Institute of Iran between 2019 and 2021 for high-dose-rate brachytherapy (HDR-BT) as primary or adjuvant therapy. Before treatment, all patients underwent comprehensive clinical assessments with complete skin exposure and relevant imaging studies to exclude nodal or visceral metastases. Selection criteria for brachytherapy included definitive treatment for T1-2 N0 tumors, and adjuvant therapy for cases with positive surgical margins or residual diseases. Patients were excluded if their lesions exceeded 1 cm in thickness, involved the periosteum or brain parenchyma, or had lymphatic or distant visceral metastases. Informed consent was obtained from all patients at admission to allow their data to be shared for research purposes. Design of the study was reviewed and approved by the institutional review board (#1400-3-248-55816) and local ethics committee (ethics code: IR.TUMS.IKHC.REC.1400.480).
Treatment customization and planning
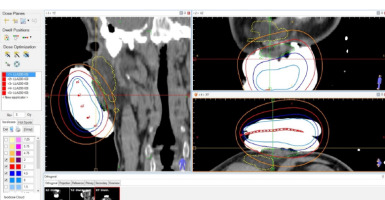
Following initial evaluations, physicians delineated clinical target volume (CTV) on the skin surface. The CTV encompassed visible tumor plus a 1-2 cm radial margin for primary cases and surgical bed with a 0-1 cm margin for adjuvant cases, depending on surgical margin status. The dedicated margin was also a function of histology and the vicinity of important facial structures. Delineation defined the periphery of the mold that was created using alginate. A metal wire was placed along the delineated area one centimeter to the mold edges, and catheters were inserted into the mold with a 1 cm spacing. Plastic tube applicators (French size 5) were utilized for post-implantation of radioactive sources. Planning involved computed tomography (CT) simulation using a 1 mm slice thickness with the mold in place. The CTV was contoured using Flexiplan software version 2.6, guided by the visible metal wire. The desired CTV depth was determined from imaging findings, with a desired maximum depth of 10 mm from the skin surface. However, some lesions were thicker than 10 mm. Paris system dosimetry was employed for treatment planning, with considerations for D90, V100, and conformity index. A radiation oncologist reviewed and verified the plan to ensure that the depth of CTV receives at least 100% of the dose, and the 200% isodose line is reasonably far from the skin surface (Figure 1). Flexitron (Elekta) device facilitated afterloading of radionuclide Co60 sources to treatment sites.
Follow-up
Patients were monitored for two years post-treatment according to guidelines from the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Acute toxicities, such as erythema and wet desquamation, were assessed during treatment as well as on day one, one month, and three months post-treatment. In contrast, late toxicities, including atrophy and pigmentation, were evaluated at six months, one year, and two years post-treatment. Follow-up assessments conducted by two radiation oncologists also focused on treatment response, cosmetic outcomes, and signs of recurrence. Suspected recurrences were confirmed through biopsy and pathological review.
The study focused on assessing primary outcomes, including local control and overall survival (OS). OS was defined as the proportion of patients surviving from the completion of radiotherapy to the end of follow-up period. Local control rate represented the percentage of patients without recurrence two years post-treatment.
Statistical analysis
Patients’ cohort was categorized into squamous cell carcinoma (SCC) and basal cell carcinoma (BCC), and the groups were compared using a χ2 test for treatment-related toxicity rates. Actuarial survival rates and curves were estimated using Kaplan-Meier method, with comparisons made between the groups with log-rank test. OS and local failure rates were calculated based on time intervals from the last radiotherapy session to follow-up end points or events. Statistical analyses were conducted using R software version 4.3.1, with significance level set at 0.05.
Results
Patient characteristics
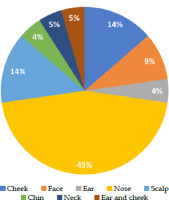
Between 2019 and 2021, 22 patients were included in the study. Of these, 18 were men and 4 were women. The mean age was 70.94 ±10.7 years. The BCC to SCC ratio was 14 : 8. The most frequent site of involvement was the nose (45.5%), followed by the scalp and cheek. Most patients (81.6%) had one lesion, and the others had multiple lesions (Figure 2).
Fig. 2
Pie chart showing the distribution of lesions’ locations in the study. The most common location for lesions was the nose, accounting for 45% of cases. The cheek and scalp each had 14% of lesions. The face had 9% of lesions, while the ear and cheek together accounted for 5%. Lesions on the chin and ear each constituted 4% of the total, and lesions on the neck also made up 5%. This distribution highlights the prevalence of lesions on the nose compared with other facial regions
Dose and planning characteristics
The size of lesions was between 0.2 and 6.7 cm, with an average of 2.46 cm. In SCC and BCC, there was no significant difference in size. The median dose was 39 Gy in 13 fractions. The median D90 was 3.04 Gy, and was almost the same in both pathologies. V100cc, V150cc, and V200cc were 12.6, 8, and 15, respectively. The distance from 100%, 150%, and 200% isodose lines to the skin surface were 9.5, 3.7, and 9 mm, respectively. V100 in SCC vs. BCC was 19.35 vs. 10.15 (p = 0.076). The CTV maximum length, CTV maximum width, and CTV maximum depth were 52, 32.8, and 10.2 mm, respectively, without significant differences between histologies. The mold characteristics are shown in Table 1.
Table 1
Technical characteristics of brachytherapy treatment for lesions with different pathologies, such as squamous cell carcinoma (SCC) and basal cell carcinoma (BCC). Data include median or mean values, interquartile ranges (IQR) or standard deviations (±SD), minimum and maximum values, and separate values for SCC and BCC lesions along with p-values for statistical comparison between the pathologies
[i] IQR – interquartile range, SCC – squamous cell carcinoma, BCC – basal cell carcinoma, D90 – dose received by 90% of the target volume, V125 – volume receiving 125% of the prescribed dose, V100 – volume receiving 100% of the prescribed dose in milliliters, V100% – percentage of target volume receiving 100% of the prescribed dose, V150% – percentage of target volume receiving 150% of the prescribed dose, V150cc – volume receiving 150% of the prescribed dose in cubic centimeters, V200cc – volume receiving 200% of the prescribed dose in cubic centimeters, V200% – percentage of target volume receiving 200% of the prescribed dose, CTV – clinical target volume
Clinical outcomes
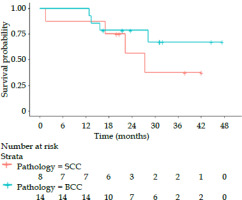
During the 30-month follow-up interval, 8 out of 22 patients died: five patients died due to cancer and three patients died due to other reasons. Among these patients who died of cancer, one had BCC (8.3%) and four had SCC (50%). Two patients had recurrences: one BCC (7.1%) and the other SCC (12.5%); thus, the 2-year local control rate was 92.9% vs. 87.5% (Table 2). Figure 3 shows Kaplan-Meier survival curves for OS based on histology. The median OS in patients with SCC and BCC was 28 months vs. not reached (p = 0.416). The 2-year OS rate was 71%, and 55% vs. 80% in SCC and BCC groups, respectively.
Table 2
Clinical characteristics and outcomes for squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) lesions treated with brachytherapy. Data include the number and location of lesions, local recurrence, last follow-up status, and cause of death, with p-values indicating statistical significance of differences between the two pathologies
Fig. 3
Kaplan-Meier survival curves illustrating the overall survival probabilities for patients with squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) over 48 months. Red curve represents patients with SCC, while blue curve indicates patients with BCC. X-axis shows the time in months, and y-axis displays the survival probability. The number of patients at risk at different time points is displayed below x-axis for each pathology. The curves indicate that patients with BCC had a higher overall survival probability than those with SCC
Discussion
Our study evaluated surface mold brachytherapy’s effectiveness and technical characteristics for treating head and neck NMSC between 2019 and 2021. Most of our cases with BCC were located in the nose skin while most of the SCC cases occurred in the scalp skin. The analysis revealed a local control rate of 87.5% vs. 91.9% for SCC and BCC, respectively, within a follow-up period of 30 months. The 2-year OS rate was 55% vs. 80% for SCC and BCC, respectively. This OS rate was expected due to the advanced age of our patient population, with a mean age of 71 years. The majority of deaths among SCC patients were due to cancer itself, whereas in BCC patients, the causes of death were mainly other than cancer-related. Our data indicated that BCC patients generally had better outcomes, although this was not statistically significant.
Potential explanatory factors for this finding include the biology of SCC vs. BCC. BCC lesions are generally slower-growing tumors, with lower metastatic potential than SCC. The inherent aggressiveness of SCC might contribute to the observed differences in outcomes. BCC often arises from the nose skin while SCC originates from the scalp skin in close proximity to the skull and bone. These anatomical differences could influence treatment response to HDR-BT. Another explanation relates to treatment planning and dosimetry. Our study mentions a trend towards higher V100 values (percentage of tissue receiving 100% of the prescribed dose) in SCC compared with BCC (p = 0.076). While not statistically significant yet, this could be explored further to observe if the dose delivered affects outcomes differentially between histologies. Differences in tumor size or depth between BCC and SCC might necessitate adjustments in treatment planning, potentially influencing outcomes.
Our findings align with previous studies, which reported local control rates ranging from 89% to 100% for head and neck NMSC treated with surface mold brachytherapy [10, 12, 19-22].
The median dose in our study was 39 Gy in 13 fractions. However, the optimal dose and fractionation regimen remains a subject of ongoing research, as both factors significantly impact treatment outcomes.
The following is a summary of how different dose and fractionation schemes can influence efficacy and toxicity.
In a similar study, Laliscia et al. retrospectively analyzed tumor control, toxicity, and esthetic outcomes in patients with NMSC treated with HDR brachytherapy using surface molds. This study included 40 lesions, predominantly BCC and located on the scalp and face. Custom-made molds were used to deliver a total dose of 40 Gy in 8 daily fractions. With a median follow-up of 25 months, the 2-year local control rate was 90%. No severe toxicities (grade 3 or higher) were reported. The cosmetic outcomes were favorable, with 65% of lesions demonstrating excellent results, and only 2.5% reporting poor cosmetic outcomes due to skin ulcerations [19].
In a study by Allan et al., a total dose of 45 Gy in 8 fractions was used for superficial carcinoma of the pinna, achieving a 100% local control rate, with no recurrences observed over a minimum follow-up of 18 months. This may suggest that even lower total doses can be highly effective for specific tumor types and locations with minimal toxicity, especially for superficial and less aggressive tumors [12].
Maroñas et al. investigated treatment of facial cutaneous carcinoma using 192Ir HDR-BT with customized molds. The treatment involved delivering 48-57 Gy in 3-4 Gy fractions, three times a week to 51 epitheliomas. After a median follow-up of 45 months, five tumors recurred, mostly occurring on the tip of the nose. The 5-year actuarial local control rate was 89%, reaching 100% for flat surfaces and 83% for nasal lesions. The treatment was well-tolerated, with 21.6% of patients experiencing severe acute toxicity; late cosmetic outcomes were rated as good or very good. Higher total doses provided robust local control rates, potentially suitable for thicker or more aggressive tumors, where a higher radiation dose is required to achieve tumor eradication [20].
Guix et al. studied 136 patients with BCC or SCC of the face, who underwent HDR brachytherapy using custom-made surface molds. Patients received 60-65 Gy in 33-36 fractions for smaller lesions, and up to 75-80 Gy for larger ones after a treatment pause. The 5-year actuarial local control rate was 98%, 99%, and 87% for all, primary, and recurrent tumors, respectively. Complete response and excellent treatment tolerance were observed in all cases, with minimal complications reported [21].
A retrospective study by Delishaj et al. used HDR-BT with Valencia applicator in elderly patients with NMSC, delivering either 40 Gy in 8 fractions (group A) or 50 Gy in 10 fractions (group B). After 12 months of follow-up, 96.25% of lesions responded completely in both groups. The acute grade 1-2 toxicities were observed in 63.2% of lesions, with a higher prevalence in group B than in group A (77.7% vs. 56.3%). Late grade 1-2 toxicities were relatively consistent between the groups (19.3%). Cosmetic results were outstanding in 86% of cases, showing a slightly higher rate in group A compared with group B (87.55 vs. 77.8%). This indicates a dose-dependent relationship with toxicity, although the adverse events in both groups were manageable, and no severe G3 or higher toxicities were reported [16]. Similarly, Arenas et al. observed increased acute toxicity rates in more fractionated regimens. However, severe toxicities (grade 3 or higher) were infrequent [10]. These studies showed lower and higher total doses, and demonstrated efficacy in achieving local control in HDR-BT for NMSC patients. Therefore, the most effective dosage should be customized to fit particular clinical situation, and maximizing tumor control while minimizing acute and late toxicities.
Several studies have been conducted to minimize acute and late toxicities. Montero et al. studied HDR plesiotherapy using custom-made molds for NMSC lesions. They employed a standard fractionation regimen delivering 44-48 Gy over 11-12 fractions for four weeks. After a mean follow-up of 15 months, excellent local control with no observed relapses was achieved. This approach spans the total dose over a longer period, potentially reducing toxicity risk. The acute toxicity was deemed acceptable, and cosmetic outcomes were excellent or good in 7 out of 9 patients [22]. Standard fractionation regimens may reduce the risk of acute toxicities and be associated with better cosmetic outcomes. On the other hand, hypofractionated regimens offer logistical advantages and reduced overall treatment times, benefiting patients’ convenience and resource management [15]. While there appears to be some controversy, both approaches effectively achieve high local control rates in treating NMSC. This highlights the versatility of HDR-BT in adapting to different clinical scenarios, while maintaining excellent outcomes.
In addition to our findings on surface brachytherapy, several studies using interstitial or contact HDR brachytherapy in other complex facial regions have been conducted, all of which are presented in Table 3. For instance, a study on eyelid NMSC lesions found that interstitial brachytherapy delivered precise doses to the CTV while minimizing radiation exposure to sensitive structures, such as the lenses. During a mean follow-up of 24 months, two patients (7%) experienced relapses: one a local recurrence in the irradiated area and the other metastases to sub-mandibular lymph nodes. This technique has proven to be an effective, short, and low-burden treatment, with no severe late complication. Thus, interstitial techniques may be suitable options for larger or more invasive lesions, which require enhanced precision [23].
Table 3
Results of various studies assessing the effectiveness and safety of contact or superficial high-dose-rate brachytherapy (HDR-BT) as well as interstitial HDR-BT with different dose and fraction schedules
| Lesions | Location | Brachytherapy schedule | Toxicity | Recurrence rate and pattern | Follow-up time | Study [Ref.] |
|---|---|---|---|---|---|---|
| 134 BCC (n = 92), SCC (n = 42) | H (mask) area: Central face, nose, and chin (n = 66) M area: Scalp, cheeks, and forehead (n = 47) L area: Trunk and extremities (n = 21) | Hypofractionated HDR-BT with fixed applicator or customized mold Mean dose: 48.79 Gy (45-54 Gy) for BCC and 50.55 Gy (45-57 Gy) for SCC, in 3 Gy fractions | Grade < 2 acute toxicity: 57.3%, grade 4 acute toxicity: 2.2% Borderline significant increase of toxicity was associated with customized molds (p = 0.067) Larger tumors were associated with higher acute skin toxicity | 2 lesions persisted after treatment Leipzig applicator used 6 (4%) lesions suffered local recurrence (Leipzig applicators: n = 5, molds: n = 3; p = 0.404) | Median: 33 months | [10] |
| 13 | Pinna | HDR-BT using surface molds, 45 Gy/8 fx., over 5 days | No late radiation complications | No local recurrences | Mean: 18 months | [12] |
| 236 BCC (n = 121), SCC (n = 115) | Head and neck (n = 198), extremities (n = 26), trunk (n = 12) | HDR-BT using Leipzig applicators | Acute skin toxicity: Grade 1: 71%, grade 2: 34% Late skin hypopigmentation changes: 5.5% | Local control rate: 98% | Median: 66 months (25-121) | [15] |
| 40 BCC (n = 23), SCC (n = 14) | Scalp (n = 16), nose (n = 7), face (n = 10), ear (n = 7) | HDR-BT using custom surface molds Median total dose: 40 Gy (range, 25-50 Gy) Modal prescribed total dose: 40 Gy/8 fx. in 48.5% of cases | Acute toxicities: Grade 1 dermatitis: 52%, grade 1 pain: 25%, grade 1 ulceration: 22%, grade 2 dermatitis: 30%, grade 2 ulceration: 5% Most common grade 1 late toxicities: fibrosis 17%, atrophy 15%, hypopigmentation 12% No grade 3+ acute or late toxicity | 2-year local control rate: 90% | Median: 25 months (3-70) | [19] |
| 51 BCC (n = 42), SCC (n = 9) | Nasal tip (n = 15), nasal pyramid (n = 15), temple (n = 9), nasal wing (n = 5), frontal (n = 5), cheek (n = 2) | Contact HDR-BT using custom molds A total dose of 48-57 Gy in 3-4 Gy fractions 3 times/week | Acute grade 1-2 dermatitis: 78%, acute grade 4 dermatitis: 21% No late complications | Local relapse: n = 5 (9.8%) all in primary tumors on the nose (nasal tip: 4, nasal pyramid: 1); 2 (4%) on the border of radiation field, and 3 (6%) inside the field 5-year actuarial local control rate: Overall: 89%, flat surfaces: 100%, nose: 83% | Median: 45 months (4-102) | [20] |
| 136 BCC (n = 102), SCC (n = 34) | Nose (n = 28), eyelid (n = 16), upper lip (n = 22), lower lip (n = 6), cheek (n = 11), ear (n = 12), preauricular (n = 22), scalp (n = 19) | HDR-BT using custom molds Minimum dose: lesions of up to 4 cm: 60-65 Gy in 33-36 fractions of 18 Gy/fx., lesions greater than 4 cm: boosted up to 75-80 Gy after a 3-week pause | Grade 1 erythema: 86%, grade 2 erythema: 14%, grade 1 ulceration: 10% No severe, early, or late complications | Local recurrence: n = 3 (2.2%): 1 SCC of frontal region, 1 recurrent BCC of cheek, 1 second recurrence of BCC of nasal columella 5-year local control rate: 99%, 5-year control rate in patients treated for recurrences: 87% | Not reported | [21] |
| 11 BCC (n = 8), SCC (n = 3) | Forehead (n = 1), eyelids (n = 2), nose (n = 3), cheek (n = 2), chin (n = 1), forearm (n = 1), hand (n = 1) | HDR-BT using custom molds; Final dose of 44-48 Gy in 11-12 fractions of 4 Gy over 4 weeks | Acute skin toxicities: Grade 1 (n = 2), grade 2 (n = 6), grade 3 (n = 3); No grade 4 toxicity Grade 2 acute conjunctivitis (n = 2) | 0% | Mean: 15 months (4-36) | [22] |
| 28 BCC (n = 24), SCC (n = 4) | Lower eyelid (n = 15), upper eyelid (n = 1), medial canthus (n = 12), lateral canthus (n = 1) | Interstitial HDR-BT 49 Gy/14 fx., 3.5 Gy BD for 7 days (n = 9), and 45 Gy/9 fx., 5 Gy BD for 5 days (n = 19) | Acute: 100%; Late: 71% | 7% (n = 2) Local recurrence in irradiated site (n = 1, BCC); Metastasis to sub-mandibular lymph nodes (n = 1, SCC) | Mean: 24 months (4-49) | [23] |
| 40 BCC (n = 10), SCC (n = 30) | Nasal vestibule (n = 26), lip (n = 10), eyelid (n = 4) | Interstitial HDR-BT Nose: 44 Gy/14 fx., 3 Gy BD (first and last fraction: 4 Gy), lip: 45 Gy/9 fx., 5 Gy BD, eyelid: 49 Gy/14 fx., 3.5 Gy BD | Most cases had grade 1-2 toxicities, with no grade 3-4 toxicity | Local recurrence: 7.5% (n = 3), two marginal recurrences (1 nasal vestibule and 1 lip), and one central recurrence (nasal vestibule) Actuarial 3-year local control: 94% | Mean: 24 months (6-40) | [24] |
| 33 BCC (n = 9), SCC (n = 20), undifferentiated (n = 4) | All lesions in ear and peri-auricular area | Contact BT (CBT) (n = 21) Interstitial BT (IBT) (n = 11) 45 Gy/15 fx. (1 fx./day), 45 Gy/9 fx. (2 fx./day, ≥ 6 h interval), 42 Gy/14 fx. (2 fx./day, ≥ 6 h interval), 20 Gy/5 fx. (1 fx./day), 21 Gy/3 fx. (1 fx./week) Intervals from 6 h (IBT) to a maximum of 7 days (CBT) Treatment duration: 1-42 days Total dose range: 7-49 Gy | CTCAE: Grade 1: n = 14 (42%), grade 2: n = 2 (6%) RTOG: Grade 1: n = 15 (45.5%), grade 2: n = 3 (9%) No grade 3+ | 3% (n = 1) Local recurrence in irradiated area | Mean: 29.75 months (2-64) | [25] |
| 102 BCC | Head and neck | Superficial 2D HDR-BT; Total dose of 50 Gy/10 fx. | Early toxicities Primary treatment group: Grade 1: 20.3%, grade 2: 28.8%, grade 3: 42.4%, grade 4: 8.5%; Recurrence treatment group: Grade 1: 16.3%, grade 2: 41.9%, grade 3: 37.2%, grade 4: 4.6% Late toxicities Primary treatment group: Grade 1: 33.9%, grade 2: 50.8%, grade 3: 1.7%, grade 4: 11.9%; Recurrence treatment group: Grade 1: 30.2%, grade 2: 62.8%, grade 3: 4.6% | Local recurrence rate: 4% Primary treatment group: 3.39% (auricle and pre-auricular region) Recurrent treatment group: 4.65% (tip of the nose and auricle region) | Primary treatment group: mean: 43.7 months Recurrent treatment group: mean: 43.5 months | [26] |
| 751 BCC (n = 534), SCC (n = 217) | Neck (n = 45), scalp (n = 195), face (n = 280), nose (n = 120), ear (n = 36), trunk (n = 45), extremities (n = 30) | Interstitial, surface, or a combination of interstitial and surface mold HDR-BT BCC group: 41.6 Gy/8 fx.; SCC group: 46.8 Gy/9 fx. | Acute toxicity: 75.9%, BCC: 75.9%, SCC: 75.9% Late toxicity: 4.8%, BCC: 1.9%, SCC: 11.8% | Local recurrence rate: n = 3, BCC (0.4%), overall local control rate: 95.6%, overall lymph node relapse rate: n = 1, SCC (0.5%), SCC regional lymph node metastasis rate: n = 3 (1.4%); SCC loco-regional control rate: 98.2%, overall loco-regional control rate: 99.5% | Mean: 36 months (1-5 years) | [27] |
| 22 BCC (n = 14), SCC (n = 8) | Nose (n = 10), cheek (n = 3), ear (n = 1), face (n = 2), scalp (n = 3), chin (n = 1), neck (n = 1), ear and cheek (n = 1) | Surface mold HDR-BT: 39 Gy/13 fx. | Acute toxicities were mild; No severe late complications | Recurrence rate: BCC: n = 1 (7.1%), SCC: n = 1 (12.5%) 2-year local control rates: BCC: 92.9%, SCC: 87.5% | 24 months | Current study |
In a retrospective study analyzing the treatment out-comes of patients with periorificial face cancers (PFC) treated with HDR interstitial brachytherapy, the 3-year local control rate was 94%. The study population predominantly presented with SCC located at the nasal vestibule, lips, and eyelids. The majority of tumors were early-stage, with T1 and T2 classifications. The treatment was well-tolerated, with no interruptions due to acute toxicity and no severe toxicities (grade 3 or 4). Mild side effects, such as edema and crusting, were common but resolved shortly after treatment. Patient satisfaction was high, with 93% of cases reporting satisfactory cosmetic outcomes. Despite three local recurrences, all successfully treated with salvage surgery, interstitial HDR-BT provided effective disease control and satisfactory cosmetic results, supporting its role as an alternative to more invasive surgical approaches for PFC [24].
Moreover, a retrospective analysis compared the effectiveness, toxicity, and cosmetic outcomes of contact and interstitial HDR-BT for lesions around the ear, where surgical challenges arise due to cartilage and esthetic concerns. The results revealed that interstitial brachytherapy carries a significantly lower risk of post-radiation reactions (9.4%) compared with contact brachytherapy (43%), making it preferable for larger and more extensive lesions, especially when the volume of healthy tissue receiving 200% and 150% doses exceeds 15 cm3 and 50 cm3, respectively. Contact brachytherapy, even though associated with higher toxicity, remains a practical outpatient alternative when interstitial therapy is not feasible. Both approaches were highly effective, with only one local recurrence and low overall treatment-related toxicity [25]. These findings underscore the need for tailored brachytherapy approach based on lesion size and anatomical complexity for achieving the optimal outcomes.
In a retrospective study on BCC tumors in the head and neck region, including both primary and recurrent cases, superficial 2D HDR-BT was assessed for efficacy and toxicity. The 5-year relapse-free survival was 96.4% for primary tumors and 94.6% for recurrent cases, with no significant difference between the groups. The toxicity profile was manageable, with early skin toxicity observed in all patients, mostly in the form of grade 2 and grade 3 reactions in approximately 30-40% of patients. Late toxicity primarily involved mild cosmetic effects, including grade 1-2 telangiectasia and pigmentation changes. Severe late toxicities, such as grade 4 skin necrosis, were rare (11.9% in the primary group, and absent in the recurrent group). The study concluded that HDR-BT is highly effective and associated with an acceptable toxicity profile, making it a valuable option for treating BCC in the head and neck region [26].
A recent larger retrospective study reviewed clinical outcomes of NMSC patients treated with HDR-BT, with majority of lesions located in the head and neck region. A total of 751 patients (534 with BCC and 217 SCC) were treated with prescription dose of 41.6 Gy in 8 fractions for BCC, and 46.8 Gy in 9 fractions for SCC. The treatment used surface custom mold, interstitial, or combination of both, depending on tumor depth. Of the total, 69% were treated with interstitial, 30% with surface custom mold, and 1% with a combination of both. The results showed a high success rate, with 96% of patients having complete responses and only 0.4% experiencing local recurrences. Local control rates were high (95.6%) and loco-regional control was even higher, especially in SCC patients, with 98.2% loco-regional control. Acute toxicities were common, with 28% of patients experiencing grade 1 toxicity, 46.7% grade 2, and 1.2% grade 3. Late toxicities were rare (mostly grade 1). Cosmetic outcomes were excellent in 79.9% of patients, good in 17.8%, and poor in only 0.5%. Overall, HDR-BT for NMSC, using all three techniques, is effective in achieving good clinical and cosmetic outcomes, with low recurrence rates and manageable toxicities [27]. Given the promising results of both surface and interstitial brachytherapy, the hybrid technique may represent a particularly advantageous option for more complex or deeper lesions to improve tumor control and minimize toxicity. This warrants further investigation as a potential optimal approach in selected cases.
We previously reported our results on HDR-BT using 192Ir source for NMSC. The 2-year actuarial local control rate was calculated to be 95% in the definitive group and 88% in the adjuvant group. After two years of follow-up, the OS rate was 87%, and 96.2% of the patients reported good/excellent cosmetic outcomes. These findings offer improved oncological and cosmetic outcomes by using HDR-BT with 192Ir, since the dose per fraction is maintained below 3 Gy per fraction [18]. In the current study, we reported our results with 60Co source. We focused more on technical aspects, suggesting that surface mold brachytherapy with 60Co source offers a practical treatment approach for the head and neck NMSC.
Recent research indicates no clinical advantages or disadvantages related to 60Co sources compared with 192Ir sources. In choosing a radioactive source in the skin, HDR-BT influences treatment planning and delivery. It is crucial to consider several factors, including tumor characteristics (e.g., size, thickness, location, and proximity to critical structures), desired dose conformity to balance efficacy with minimizing toxicity to bone marrow and other surrounding tissues [28, 29], treatment logistics to balance treatment duration with source availability and replacement requirements, cost consideration, and treatment time [30]. Generally, 192Ir is the most widely used source due to its established track record and favorable physical properties, although 60Co sources have potential logistical benefits because of their higher average energy and longer half-life, which make them a compelling option, particularly in developing nations [30-33]. Table 4 compares the characteristics of 192Ir and 60Co sources.
Table 4
Summary of characteristics of cobalt-60 (60Co) and iridium-192 (192Ir) as potential sources for high- dose-rate (HDR) brachytherapy, highlighting their half-lives, energy levels, advantages, and disadvantages
| Source | 60Co | 192Ir |
|---|---|---|
| Half-life [30] | 5.27 years | 74 days |
| Energy [32, 33] | 1.25 MeV | 0.38 MeV |
| Advantages | Longer half-life: Provides flexibility in treatment scheduling due to less frequent source replacements | Established track record: Extensive experience with 192Ir in skin brachytherapy, leading to well-defined treatment protocols Versatility: Offers a good balance between tissue penetration and dose conformity, suitable for various tumor sizes and locations Lower energy emissions: A shallower dose fall-off is potentially beneficial for treating superficial lesions and minimizing the dose to deeper structures Reduced shielding needs: Lower energy emissions translate to less stringent shielding requirements, potentially reducing treatment facility costs |
| Disadvantages | Higher energy emissions: Deeper dose fall-off, potentially increasing the risk of toxicity to deeper structures Shielding requirements: High-energy emissions necessitate significant shielding, impacting treatment facility, design, and cost | Shorter half-life: Requires frequent source replacements, impacting treatment logistics, and potentially increasing costs Limited tissue penetration: Lower energy emissions limit its effectiveness for treating thicker lesions |
Several studies have been conducted on techniques to deliver high-dose radiation more precisely, especially for treating complex, irregular, and curved surfaces. For instance, a survey of topographic applicator brachytherapy (TAB) in NMSC lesions reported manageable acute and chronic toxicity in 10.6% of cases and a low recurrence rate of 4.8%, indicating that precise application methods can effectively treat NMSC with minimal adverse effects [34]. Moreover, another innovative method is using custom 3D-printed applicators for brachytherapy, designed to conform to the unique contours of a patient anatomy. A recent study showed that these low-cost, patient-specific applicators improved fitting and dose delivery while minimizing exposure to surrounding organs at risk. This approach underscores the potential for personalized solutions in enhancing radiation therapy precision [35].
One challenge in patient selection for surface mold brachytherapy is the optimal thickness of tumor or coverage depth of desire. It has been demonstrated that smaller treatment areas and increased treatment distances significantly influence dose fall-off and local high-dose regions. For instance, a 16% reduction in dose was observed when the distance increased from 10 to 20 mm, highlighting the necessity of adjusting treatment distance to achieve optimal tumor-to-skin dose ratios [36]. The American Brachytherapy Society (ABS) consensus for skin brachytherapy indicates that molds can be considered for tumors up to 5 mm deep, and tumors more profound than 5 mm should be scheduled for interstitial brachytherapy or EBRT [37]. However, recent advancements in multilayer intensity-modulated contact interventional radiotherapy (IRT) provide a promising alternative for treating thicker NMSC lesions. A study employing a multilayer arrangement of catheters demonstrated significantly enhanced CTV coverage, achieving a V95 (CTV) of 95.68% compared with just 81.84% using standard configurations. This innovative approach allows for effective treatment of the tumors exceeding 5 mm in thickness, while minimizing excessive radiation exposure to the surrounding healthy tissue [38]. Moreover, our study utilized an innovative technique for several patients (Table 1, CTV maximum depth): thicker molds to confine the 200% isodose line within the mold strategically. This approach is particularly beneficial for treating thicker tumors, as it minimizes excessive radiation exposure to the skin while ensuring complete tumor depth coverage by the 100% isodose line for effective treatment, demonstrating a potential strength of our study.
The current study’s further strengths include employing a retrospective cohort design, which is suitable for investigating treatment outcomes, such as local control and survival in patients with NMSC treated with HDR-BT. Additionally, we established clear inclusion and exclusion criteria for patient selection, ensuring a focused study population. We had some compelling findings. The 2-year local control rate of 92.9% suggests that HDR-BT effectively controls NMSC. The overall 2-year survival rate of 71%, with potentially even higher rates for BCC, indicates promising survival outcomes. Moreover, including detailed dosimetry parameters (D90, V100, etc.) provides valuable insights into treatment planning and potential future optimization.
It is important to acknowledge the study’s limitations as well. The retrospective nature limits the ability to establish causality between treatment and outcomes. Furthermore, the relatively small sample size restricts the generalizability of findings. Also, we did not assess the toxicities of treatment and late effects in this study. Future prospective studies with larger patient cohorts are warranted to confirm these results.
Conclusions
Our study demonstrates that surface mold brachytherapy is an effective and safe treatment option for head and neck NMSC patients. The observed local control rates are comparable with other treatment modalities, showing insignificant late complications. This minimally invasive approach offers potential benefits for cosmetic outcomes, particularly in cosmetically sensitive head and neck regions. Further research with larger patient populations is recommended to solidify the role of surface mold brachytherapy in managing head and neck NMSC.
Future research directions
Stratifying the analysis by tumor size, depth, and location to investigate if the observed trends in local control and survival hold true within sub-groups based on histology.
Investigating whether BCC and SCC respond differently to variations in HDR-BT treatment parameters, such as fractionation or dose prescription.
Conducting a more extensive prospective study to confirm the initial findings and assess the long-term impact of HDR-BT on survival outcomes, stratified by histology.