Purpose
Non-small cell lung cancer (NSCLC) accounts for approximately 80-85% of all lung cancers [1]. For operable peripheral NSCLC, current guidelines recommend the use of surgical treatment combined with chemotherapy, radiotherapy, and/or other multidisciplinary treatment modalities, with the basic treatment strategies gradually transitioning to radical concurrent chemoradiotherapy or neoadjuvant therapy, followed by radical surgery and other comprehensive treatment regimens. Theoretically, neoadjuvant radiotherapy can safely reduce the tumor stage and volume, increase resectability, and reduce the possibility of tumor cells spreading and colonizing during surgery [2]. High-dose-rate brachytherapy can cause fatal and desirable damage to tumor cells. After killing tumor cells, it induces the expression of tumor-associated antigen, disrupts the local inflammatory microenvironment, stimulates effector T-cell reactivation, and remodels cellular immunity, thereby exerting a distant anti-tumor effect on regional and distant metastases [3,4,5,6,7,8].
The primary objective of this preliminary study was to examine the safety and feasibility of using stereotactic ablative brachytherapy (SABT) as a neoadjuvant therapy for a small cohort of patients with operable NSCLC. The secondary objectives of this study were as follows: 1) To evaluate the efficacy of the neoadjuvant therapy by comparing parameters obtained by positron emission tomography/computed tomography (PET/CT) and dynamic contrast-enhanced computer tomography (DCECT) (tumor volume, maximum standardized uptake value – SUVmax, and volume of blood flow), and 2) To compare the consistency of dosimetric parameters between the preoperative simulated plan and the actual delivered SABT treatment plan using the coplanar template guidance technique.
Material and methods
Patients
This study was approved by the Research Ethics Committee and was registered before initiation. Written informed consent was obtained from all patients before treatment. Patients aged over 18 years who were confirmed as having NSCLC (stage I, II, or IIIA) by pathological biopsy and were considered to be acceptable for surgical resection before enrollment were included. All the patients had the Eastern Cooperative Oncology Group performance status score of 0 or 1 (on a 5-point scale, in which higher numbers reflect greater disability).
Study design
The patients received template-assisted SABT, with a risk-adapted fractionation schedule (30 Gy delivered in one fraction). This dose was recommended by previous clinical trials [9], and surgical resection was scheduled to resect tumors 4-6 weeks after SABT. The primary endpoints were safety and feasibility. The key secondary and exploratory endpoints were the efficacy of SABT as neoadjuvant therapy and the value of the template-guidance technique.
SABT process
Positioning was simulated using a CT-sim (Light-Speed Plus 4, General Electric Company, Chicago, IL, USA), and a mechanical arm was used to guide the coplanar template to cover the tumor surface location; vacuum bag cushion was used to keep the patient in the same position. The CT images were sent to the three-dimensional (3D) radiotherapy planning system (TPS) (Oncentra 4.3, Elekta, Sweden). All DICOM data were transferred to Oncentra 3D-TPS via the local area network. After fusion with the PET images, the gross tumor volume (GTV) and organs at risk (OARs) were delineated by the attending physician. Then, the physician created the simulated-preoperative plan (SPP), which was just a simulation and was not implemented on the patients. The specific implantation number and depth of the implant needles as well as the dwell position and duration of the 192Ir source should be optimized according to GTV location. Needles were positioned at 1-cm to 1.5-cm intervals and surrounded the entire lesion. Disinfection, sterile drape spreading, and topical infiltration anesthesia were then performed. The needles were implanted according to the preoperatively planned needle path and depth. Screw caps were used to fix the implant needles in place. Details are provided in Figure 1. The CT image was then transmitted to the Oncentra planning system for the actual-operative plan (AOP), and requirements were the same as those for the SPP (Figure 2). SPP and AOP were completed on the same day. After AOP was reviewed and approved by the attending physician, the patients were sent to the treatment room for brachytherapy. Immediately after radiotherapy completion, the implant needles were removed, and needle implantation site was wrapped with compression; a chest CT scan was performed again. After excluding pneumothorax and hemothorax, the patients were returned to the ward to be observed for at least 24 hours.
Fig. 1
Details of stereotactic ablative brachytherapy (SABT) process. A) shows a mechanical arm was used to guide the coplanar template. The mechanical arm, fixed on the patient transport sled, could move in the left-right, head-foot, and anterior-posterior direction, and guide the template at any angle. The angle of the template displayed in real time on the tablet by means of a sensor mounted inside the front end of the arm. B) shows the template used, which was made of resin material. The thickness of template was 2 cm; the distance between the needle-holes on the template was 0.5 cm. The template was sterilized with ethylene oxide sterilizer before SABT. C and D) show one patient during simulated-preoperative plan (SPP) scan and before implementation of actual-operative plan (AOP). Vacuum bag cushion was used to keep patient in the same position. The angle displayed on the tablet showed that the template remains the same in SABT process
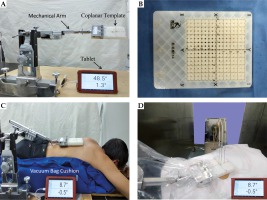
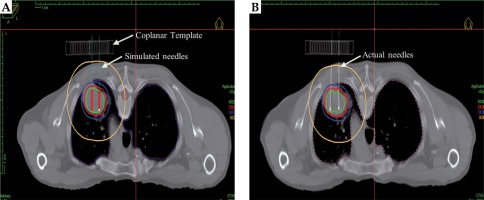
Fig. 2
Representative dose distributions for one patient for simulated (A – simulated-preoperative planning) and delivered (B – actual-operative planning) stereotactic ablative brachytherapy (SABT) plans. The coplanar template, simulated needle track, and actual implant needles have been marked with arrows. The green, red, blue, and yellow lines represent 45 Gy, 30 Gy, 20 Gy, and 5 Gy isodose curves, respectively
Assessment of safety and feasibility
Acute or late adverse reactions were estimated by referencing the Toxicity Criteria of the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer. Feasibility was prospectively defined as any delay in the planned surgery for ≤ 30 days.
Assessment of neoadjuvant efficacy
Patients underwent PET/CT and DCECT imaging before both SABT and surgery, and the results of the two scans were compared. The comparison included the following: 1) tumor volume (VGTV); 2) SUVmax; 3) tumor blood volume (TBV); and 4) tumor blood flow (TBF). For the analysis of the four types of data, if the difference was statistically significant, SABT was considered as having a significant treatment effect. Treatment response was evaluated according to the Response Evaluation Criteria in Solid Tumors (version 1.1).
Assessment of dosimetric parameters
We referred to several dosimetric parameters, such as V5, V20, and mean lung dose (MLD), of radioactive seed implantation and external radiation therapy as the evaluation indicators. Doses were converted to the biologically equivalent dose (BED) by applying the linear-quadratic model, using an α/β ratio of 10 Gy for the GTV and 3 Gy for the lungs [10,11,12].
1. Quality parameters of planning: with reference to the quality standards for evaluating external radiation treatment plans, we calculated the heterogeneity index (HI) and conformity index (CI).
HI are used to evaluate the uniformity of the dose distribution. The calculation formula [13] is as follows:
where VGTV-REF is the prescription dose volume received by GTV and VGTV-1.5REF is GTV volume after receiving 1.5 times the prescribed dose; the ideal HI value is 1, and as the plan becomes less uniform, the HI value decreases.
CI was used to describe the degree of conformity of dose distribution. Its calculation formula [14] is as follows:
where VGTV is GTV volume and VREF is the total volume contained in the prescribed dose; the CI value is between 0 and 1. A CI closer to the ideal value of 1 means a higher coverage for the GTV, and a radiation treatment plan with a CI higher than 0.60 is considered an optimal treatment plan [15].
2. GTV parameters: D90 (BED), the percentage volume when receiving 100% and 150% of the prescribed dose (V100, V150).
3. Lung irradiation dose: V5 (BED), V20 (BED), and MLD (BED).
Statistical analysis
Side effects, adverse events, and feasibility were continuously monitored. We assumed that SABT would not be feasible if the probability of surgery being delayed by at least 30 days in > 25% of the patients. Here, we used SPSS version 20.0 software (IBM Corp., Armonk, NY, USA) and the paired t-test for comparing between the two groups. Experimental data are expressed as the mean ± standard deviation (SD), and p values are two-sided, with the significance level set at 0.05 for all analyses, unless otherwise noted.
Results
Patient and treatment characteristics
This report included a total of 16 patients recruited during the period from January 2018 to June 2018. SABT was performed without issues in all patients. The relevant clinical characteristics of the patients and treatment details are presented in Table 1.
Table 1
General conditions of patients and details of implanting technique
Safety and feasibility
The perioperative adverse events are summarized in Table 2. Patients did not experience any serious adverse events. Three patients experienced minor complications (one patient had blood in the sputum and did not require any active intervention; one patient had a small amount of pneumothorax and recovered spontaneously; one patient had a hematoma in the lungs with a hematoma scope of 2 × 3 cm, which resolved after symptomatic hemostatic treatment). None of the patients had surgical contraindications at 4-6 weeks after SABT and were able to undergo surgery as scheduled.
Neoadjuvant efficacy
VGTV, SUVmax, TBF, and TBV changes from pretreatment to 4-6 weeks after SABT are detailed in Table 3. The pre-surgical primary GTV volume was significantly smaller than the pre-SABT primary GTV volume. None of the patients had CR (complete response), nine had PR (partial response), five had MR (minor response), and two had SD (stable disease). The reductions in the primary GTV of three patients were > 50% (Figure 3). The pre-surgery values were significantly lower than those pretreatment for SUVmax, TBF, and TBV.
Table 3
VGTV, SUVmax, TBF, and TBV for the primary GTV
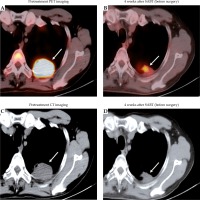
Fig. 3
Patterns of radiologic response to neoadjuvant therapy with stereotactic ablative brachytherapy (SABT). PET and CT images of the chest of a 63-year-old male smoker, with stage IIB non-small cell lung cancer (NSCLC) before (A, C) and after (B, D) the neoadjuvant SABT are shown. In the left panel, the pretreatment scan shows a primary tumor measuring 4.2 cm in diameter (SUVmax = 14.20) in the left lobe of the lung (arrow). In the right panel, a scan performed 4 weeks after SABT (but before surgery) shows shrinkage with a 52% associated tumor cavitation (arrow) (SUVmax = 4.76)
Dosimetric comparison of SPP and AOP
According to a comparison between SPP and AOP, the dosimetric parameters for the GTV and the lung had slight changes, but there was no statistically significant difference (p > 0.05) (Table 4).
Table 4
Comparison of dosimetric parameters between the simulated preoperative plan and the delivered SABT plan (mean ± standard deviation)
[i] GTV – gross tumor volume, SABT – stereotactic ablative brachytherapy, BED – biologically equivalent dose, MLD – mean lung dose, GTV V100, V150 – percentage volume of the GTV receiving 100% and 150% of the prescribed dose, Lung V5, V20 – percentage volume of the lung receiving 5 Gy and 20 Gy, HI – heterogeneity index, CI – conformity index, SPP – simulated preoperative plan, AOP – actual operative plan
Discussion
Neoadjuvant radiotherapy has become a treatment option for many cancers (e.g., rectal cancer), with the goal of reducing the risk of local recurrence and improving resectability [16]. A retrospective study published by Glover et al. [17] found that neoadjuvant therapy did not increase intraoperative blood loss, operation duration, or in-hospital mortality rate compared with surgery alone, and that the total perioperative complication rate did not differ significantly between study groups. Current studies suggest that brachytherapy has now become a safe and effective treatment for inoperable early-stage NSCLC [18]. However, this combination of brachytherapy as a neoadjuvant therapy for early-stage, operable NSCLC has not been reported. We observed that the neoadjuvant treatment of SABT in patients with early-stage lung cancer was associated with few acute adverse events and did not cause a delay in any of the planned surgeries. Therefore, it is possible that SABT can be used as a preoperative neoadjuvant therapy for patients with early-stage NSCLC. Here, patients had significant reductions in the VGTV, SUVmax, TBV, and TBF at 4-6 weeks after SABT. In fact, eight out of 13 patients exhibited > 50% decrease in tumor volume, indicating that the efficacy of SABT as a neoadjuvant therapy was significant.
The implant needles serve as a channel for the 192Ir source to enter the tumor. Their implantation position, angle, and depth are important factors that affect the quality of SABT. It is difficult to accurately insert the implant needles according to the preoperative simulated plan by simply relying on image-guidance technology. Moreover, due to respiratory motion, obstruction by the ribs, and the need to avoid important anatomical structures, frequent adjustments are made to the implant needles’ positioning during the implantation procedure, which increases the possibility of complications. How to accurately and effectively insert the implant needles according to the preoperative simulated plan is one of the main challenges of brachytherapy for lung cancer treatment. Compared with purely manual operation, the use of coplanar template guidance technology not only reduces the dependence on the operator’s personal experience, but also improves the operating efficiency, while also avoiding possible discomfort and complications associated with repeat adjustments of the implant needles as well as avoiding repeat CT scans. Additionally, the coordinate system established using the coplanar template determines the target area of the tumor and provides information on the position and depth of each needle; this ensures that the implantation position, angle, and depth of each needle as well as the position of the 192Ir source in the needle canals are consistent with those of the preoperative treatment plan. A study by Yang et al. [19] reported that the irregular arrangement of the implant needles limits further reductions in lung radiation dose during brachytherapy for lung cancer. In this clinical study, a coplanar template guidance technique was used to ensure that the implanted needles were kept parallel to each other without offsets or crossover. These findings show that SABT can effectively ensure lung radiation dose, reduce the incidence of radiation pneumonitis and other complications, and reduce the perioperative risks of surgery.
Our study has some limitations. The sample size considered in this interim report is small, and our study was non-random. Moreover, the evaluation of parameters for neoadjuvant therapy may have been insufficient, and therefore caused a probable bias. However, these data are still informative. They sufficiently assure us that it is safe to continue the present clinical trial involving a combined treatment, which also provides reassurance to other groups who are designing or conducting similar trials.
Conclusions
Although these preliminary results are not sufficient to support wide clinical application, they do provide an early evaluation of the safety and feasibility of SABT as a neoadjuvant therapy in patients with operable NSCLC. Additionally, it was validated that there was no statistically significant difference in the dosimetric parameters in either the GTV or the OARs between the simulated and delivered treatment plans. Therefore, we believe that the coplanar template guidance technique can ensure good consistency between the simulated and delivered SABT plans, which provides an effective tool for delivering brachytherapy for lung cancer.
Declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of Affiliated Hospital of Southwest Medical University and in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study.
Trial registration
Chinese Clinical Trials Register No. ChiCTR1800014541. Registered 20 January 2018, http://www.chictr.org.cn/showproj.aspx?proj=24870.





