Purpose
In 2017, the GEC-ESTRO (Groupe Européen de Curiethérapie – European Society for Therapeutic Radiology and Oncology) published an update of its recommendations for head and neck (HN) brachytherapy. This update focused on the implementation of cross-sectional imaging-based treatment and stepping source technology. The guidelines addressed various topics, such as dose and fractionation, selection of brachytherapy for different treatment indications, quality assurance, and physical aspects [1].
Since its publication, advances in the understanding and management of head and neck cancers have influenced the practice of brachytherapy [2, 3]. The present scoping review aimed to depict the evolution of HN brachytherapy research and practice as reflected in published literature from 2017 to 2023, and to identify emerging topics since the previously published recommendations.
Material and methods
Our methodology and results were reported according to the preferred reporting items for systematic reviews and meta-analyses extension for scoping reviews (PRISMA-ScR) [4].
Systematic search
A systematic literature search was performed in PubMed, EBSCOhost, Europe PMC, and Google Scholar databases for articles on HN brachytherapy. Search strategies included the following: “SU brachytherapy AND SU (head and neck cancer)” for EBSCOhost, “(brachytherapy[MeSH Major Topic]) AND (head and neck cancer[MeSH Major Topic])” for PubMed, “(KW:“brachytherapy” AND KW:“head and neck cancer”)” for Europe PMC, and “allintitle: brachytherapy AND “head and neck” OR nasopharynx OR nose OR nas OR oropharynx OR oral OR orbit OR lip OR buccal OR tongue OR lingual OR neck OR sinus OR sino OR maxilla” for Google Scholar. A filter was applied for articles published from the year 2017 onwards. The last search was done on June 29, 2023.
Study selection
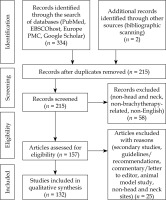
Clinical trials, prospective and retrospective cohort studies, cross-sectional studies, case series, case reports, and dosimetric and simulation studies were included. Dosimetric studies were those reporting on doses from treatment plans that were actually delivered to patients; simulation studies were those that used image datasets from brachytherapy cases or phantoms, and reported on simulated doses based on hypothetical dose regimens that were not delivered to patients. Letters, editorials, and commentaries were excluded. Bibliographies of guidelines and systematic reviews were scanned for other relevant titles. Only studies in English were included. Each study was screened by one of four reviewers, and checked by a second. Any disagreement was resolved by discussion with other reviewers (Figure 1).
Data extraction and charting
Four reviewers performed data extraction. The final list of screened articles was equally divided between two sets of independent reviewers, with each pair working on a designated subset. In case of a conflict or discrepancy, a third reviewer would arbitrate.
Study details were extracted from articles using a standardized template. An iterative team approach to data charting (categorization, extent of detail) was employed, beginning with five pilot charts for each reviewer. In the last iteration, the following data were tabulated: HN subsite, other topics discussed (e.g., re-irradiation, nursing, physics, training), publication year, treatment period (if applicable), origin of publication, study design, number of patients, dose-rate (i.e., LDR, pulsed-dose-rate [PDR], high-dose-rate [HDR]), implant technique (i.e., intracavitary, interstitial, mold, combined), implant approach (i.e., free-hand, template-guided), implant guidance (i.e., visual-guided, palpation-guided, imaging-guided), treatment setting (i.e., definitive, post-operative, perioperative), method details reported, outcomes reported, and analyses performed. Given the objective, no formal risk of bias appraisal was done [5].
Data analysis
Quantitative analysis was completed with descriptive frequency counts of the tabulated entries for each category of extracted information. Qualitative content analysis was performed using a method described by Hancock [6]. A narrative synthesis on the identified emerging themes was formulated and discussed in the context of topics in the GEC-ESTRO 2017 recommendations.
Results
This systematic search and bibliographic scanning yielded 215 unique articles, including one guideline and six systematic reviews. A total of 132 primary studies from 2017 to 2023 were included.
Temporal and geographical trends
In this period, there was no noticeable upward or downward trend in the number of publications on HN brachytherapy, with an average of 21 publications per year from 2017 to 2022. More than two-thirds of the publications came from China, the United States of America (USA), India, and Japan (Table 1). China consistently produced the most research output per year from 2017 to 2022. Since 2020, an increasing proportion of published studies originated from Japan.
Study types
A total of 112 studies reported clinical outcomes, including four clinical trials, 17 prospective cohorts, 72 retrospective cohorts, four case series, and 15 case reports. Of these, 89 studies reported outcomes of cohorts treated in the 2000s and 2010s. The largest number of patients included in a single report was a retrospective cohort from the USA, with brachytherapy outcomes for base of tongue carcinoma using national registry data of 15,934 patients treated from 2004 to 2012 [7]. A total of 22 articles included simulation (n = 11), dosimetric (n = 7), and physics (n = 4) studies.
Clinical studies
Among clinical research, the most studied sites were the oral cavity (n = 84), oropharynx (n = 37), and salivary glands (n = 20). The subsites studied are detailed in Table 2. Furthermore, 21 studies investigated re-irradiation using brachytherapy, 5 pediatric brachytherapy, and 10 combination with other modalities, such as external beam irradiation, chemotherapy, chemoradiation, or pre-operative trans-arterial chemoembolization.
Table 2
Sites and subsites studied
Where dose-rate was specified, HDR (n = 57) or LDR (n = 50) brachytherapy were predominantly investigated. Four studies reported PDR brachytherapy, and six a combination of two or all the above. Most LDR studies explored permanent seed implants (PSI) using 125I (n = 39). Majority of HDR or PDR studies used 192Ir (n = 34). Others reported the use of 198Au (n = 8), 131Cs (n = 5), 60Co (n = 2), 103Pd (n = 4), 252Cf (n = 1), and 224Ra (n = 2).
The treatment setting as well as implant technique, approach, and guidance described in the studies are summarized in Table 3. Brachytherapy was applied in definitive, post-operative, or perioperative settings in 78, 33, and 17 studies, respectively. Interstitial, mold, and intra-cavitary techniques were described in 105, 13, and 5 studies respectively. Five studies also utilized a combination of these techniques. Free-hand approach was used in 88 studies, and template-based approach in 27. Nearly all studies used image-guided methods (n = 52), mostly PSI studies. Of these, 50 were CT-guided and 2 ultrasound-guided. Other studies described visual (n = 57) or palpation (n = 25) guidance.
Table 3
Treatment setting, and implant technique, approach, and guidance
Pre-clinical studies
Among pre-clinical studies, 11 discussed 3D-printing and 6 reported applicator design. Two physics papers discussed new approaches to dose calculation and dose optimization algorithms. Two studies investigated the use of novel radioactive sources, such as 252Cf (n = 1), 75Se (n = 1), 169Yb (n = 1), and 153Gd (n = 1). Other pre-clinical studies explored 198Au (n = 1), 131Cs (n = 2), and 60Co (n = 1).
Discussion
This scoping review investigated emerging themes in HN brachytherapy publications in the last seven years, which were identified from the frequency counts of the extracted data. We now discuss these emerging themes in relation to the existing recommendations from the GEC-ESTRO [1]. Table 4 summarizes the main points of the discussion.
Table 4
Emerging topics in relation to areas addressed in the 2017 GEC-ESTRO recommendations
| Topic | GEC-ESTRO recommendations (2017 update) [1] | Emerging interests (2017-2023) |
|---|---|---|
| Fractionation schedules | Schedules for HDR and PDR brachytherapy (transitioning from LDR wires) | Increasing literature on LDR permanent seed implant may provide evidence for recommendations on dosimetry and treatment planning |
| Brachytherapy use in specific subsites | Discussed primary brachytherapy in lip, oral cavity, oropharynx, nasopharynx, and superficial cancers | Emerging data on the utilization of interstitial seed brachytherapy for parotid cancers |
| Adjuvant brachytherapy | Predominantly delivered post-operatively, with intra-operative and pre-operative brachytherapy considered investigational | Increasing literature on the use of perioperative brachytherapy, with one study reporting longer follow-up (10-year recurrence and survival rates) |
| Physics | Reported on implant checking, treatment planning, dose calculation, and treatment delivery | Initial data from simulated plans generated from inverse planning algorithms, and on the performance of a model-based dose calculation algorithm |
| 3D printing | Not discussed | Several articles discussing 3D printing for template and applicator design |
Low-dose-rate permanent seed brachytherapy
The latest update of the 2017 GEC-ESTRO recommendations discussed general aspects of treatment planning, including target volume definition, treatment planning parameters with current stepping source systems, and fractionation schedules for HDR and PDR brachytherapy [1]. Recent publications suggest a resurging interest in LDR brachytherapy, particularly in the form of PSI, as shown by the growing number of studies on this technique (n = 50). It was applied in the definitive or post-operative treatment of locally advanced or inoperable cancers [8-10], early tongue cancers [11], parotid malignancies [12-17], and minor salivary gland carcinomas of the lip and buccal mucosa [18]. Details on the methods of implantation, dosimetric planning, and treatment delivery are described in these studies. Iodine-125 seeds were mostly used, with prescribed doses ranging from 60 Gy to 160 Gy in the mentioned articles. Various reports also described the utility of LDR seed brachytherapy in the setting of re-irradiation or recurrent tumors. Doses applied in these studies ranged from 90 Gy to 160 Gy using 125I seeds [19-24], and from 40 Gy to 70 Gy using 131Cs seeds [25-27]. To our knowledge, there are no current specific guidelines for this modality. Further analysis of data from methodologies and outcomes of these studies may provide evidence for future recommendations on implantation, dosimetry, and treatment planning.
Subsites
The GEC-ESTRO guidelines included discussions regarding the role of primary brachytherapy in malignancies, such as lip, oral cavity, oropharynx, nasopharyngeal, and superficial cancers [1].
Several retrospective studies in this review reported the application of brachytherapy in salivary gland malignancies, particularly parotid cancers. As mentioned previously, several studies on LDR PSIs were performed on this site, and patients were treated both in definitive and post-operative setting. There are articles reporting LDR as an effective primary treatment in the definitive setting without causing severe complications [12, 17]. These findings indicate that there may be emerging data on utilization of LDR seed brachytherapy for parotid cancers, which warrant further investigations.
Perioperative brachytherapy
In addition to discussion on brachytherapy as a primary modality of treatment, the 2017 GEC-ESTRO recommendations also tackled the role of adjuvant brachytherapy. Most of the discussion focused on the use of post-operative brachytherapy performed 1-2 months after surgery [1].
While post-operative brachytherapy remains the more common adjuvant procedure in recent literature (n = 33), studies on perioperative brachytherapy are also growing. With some publications reporting longer follow-up, these additional data can add to the current evidence on its oncologic outcomes and toxicity. For example, acceptable six-year loco-regional outcomes of its use in early mobile tongue cancer was reported [28]. Also, in Khan et al. study, ten-year data on recurrences and overall survival rates were described on its application in the salvage setting for neck recurrences, showing encouraging results and relatively low toxicity rates [29].
Salvage brachytherapy and re-irradiation
The 2017 recommendations recognized salvage brachytherapy as a treatment option in previously irradiated patients. A continued interest in salvage brachytherapy in the setting of re-irradiation was seen, as more studies in recent years (n = 21) further investigated its role. Procedures employed different techniques using HDR, PDR, or LDR brachytherapy, and were performed in different HN sites. In addition to several case reports and retrospective studies, recent prospective data were delivered on this topic. In a study by Martínez-Fernández et al. on 63 patients, perioperative HDR brachytherapy in addition to surgery resulted in long-term loco-regional control, with a 5-year loco-regional control rate of 55% [30]. However, the authors observed significant rates of toxicities, with 50.8% of patients experiencing at least grade 3 adverse effects. Luginbuhl et al. enrolled 49 patients in a prospective study using intra-operative 131Cs seed brachytherapy. They demonstrated comparable outcomes in comparison with historical cohorts and acceptable safety profile. Two-year disease-free survival was reported in 49%, and rates of osteo-radionecrosis and percutaneous endoscopic gastrostomy (PEG) tube placement were low [26].
Physics
The discussion on general quality assurance and physical aspects in the 2017 GEC-ESTRO recommendations described practical guidance on the procedure of implant checking, treatment planning, dose calculation, and treatment delivery. Recent studies on the physical aspect of brachytherapy included some dosimetric or simulation research. One study compared the dosimetric results, total dwell time, and number of active positions between plans generated by inverse planning simulated annealing (IPSA) and hybrid inverse planning and optimization (HIPO). They observed comparable dosimetric results between the two algorithms, and a benefit of shorter dwell time using HIPO [31]. Another study determined the performance of advanced collapsed-cone engine (a model-based dose calculation algorithm) in treatment planning for scalp brachytherapy. This was compared with the Task Group 43 (TG-43) dose calculations, in which similar doses were found above the skull layer of the phantom, while an underestimation of the dose through the bone was observed [32].
Applicator design and 3D printing
Applicator design and 3D printing were also among the major themes in simulation studies found in the current review [21, 33-36]. These studies described in detail the process of applicator design and fabrication. No specific recommendations have been provided on this topic yet, but emerging interest in this practice may deliver insights on its value.
For general HN cancer sites, brachytherapy using collagen matrix tiles with 131Cs was investigated in a cadaveric study, and reported feasibility, ease of use, and less carotid dose [33]. For nasopharyngeal cancers, a novel applicator design for intra-cavitary brachytherapy was proposed. A simulation study compared the dosimetric outcomes with the Rotterdam nasopharyngeal applicator, and demonstrated significantly lower soft palate doses with the new design [34].
Various studies also investigated the application of 3D printing in the fabrication of templates and applicator guides. A 3D-printed patient-specific applicator guide for oral tongue cancers was used in a phantom study. Insertion time, geometric accuracy, and dose-volumetric analysis were reported, showing improvement in the treatment process, catheter positioning, and dose homogeneity [35]. There were also retrospective studies on 3D-printed templates in patients who underwent 125I seed brachytherapy for recurrent tumors [21, 36]. The utilization of 3D-printed templates resulted in improvements in dosimetry and positioning, with no obvious adverse reactions. Similarly, 3D-printed templates were used for seed implant brachytherapy in cervical node metastases, and resulted in accurate positioning without complications [37].
Another study on personalized brachytherapy involved the use of a 3D-printed anthropometric phantom and lead shielding for the eyes in facial surface brachytherapy procedures. The study aimed to verify the doses to critical organs by measuring the calculated and measured doses, and reported using lead shield as a method for protection of organs at risk [38].
Limitations
This study has limitations inherent to the nature of scoping reviews. We aimed to include a large comprehensive body of literature to determine recent trends in HN brachytherapy research. Due to its broad scope, the depth of analysis of outcomes was limited, and critical appraisal was not done on each of the included studies to assess the quality of evidence. Generating evidence, as a basis for standard clinical practice, was beyond the scope of this review. However, the emerging topics identified in this study may direct further investigations, systematic reviews, or meta-analyses, which can serve as basis for future recommendations. Moreover, relevant studies may have been missed due to the exclusion of non-English publications, especially considering the abundance of HN brachytherapy studies coming from regions where English is not the primary language.
Conclusions
In summary, this scoping review identified recent trends in HN brachytherapy, such as the use of LDR seed implants, its application in other HN sites, perioperative brachytherapy, and 3D printing in template design. Data from these recent publications can provide a foundation for further reviews and investigations, which can generate evidence for succeeding guidelines in HN brachytherapy.