Purpose
Post-operative brachytherapy, also called interventional radiotherapy, as exclusive treatment in intermediate- and intermediate-high risk patients with endometrial carcinoma is an effective treatment for reducing the development of vaginal relapses to < 3.5% [1]. In vaginal cuff treatments, several schedules have been used in the literature, e.g., Harkenrider et al. in 2016 described 24 different schedules [2]. In the low-dose-rate (LDR) era, doses were more homogeneous, and the length of vagina treated was 3 cm to 5 cm, i.e., the upper 1/3 or 1/2 of vagina, depending on the center [2]. Subsequently, pulsed-dose-rate (PDR) and high-dose-rate (HDR) became available. Considering aspects, such as outpatient therapy and technical facilities, HDR became the most extended treatment worldwide, not only for vaginal cuff brachytherapy (VCB). The beginnings of using HDR in the last century have led to a conclusion that in order to avoid vaginal complications, the lower the dose, the higher the number of fractions. While using LDR remote afterloading machines has decreased, the use of HDR units has increased. However, in a 3D planning era, the number of fractions and doses varies greatly, not allowing for differentiation between treatments. At present, a more homogeneous scenario is necessary since the results of treatments have only been evaluated retrospectively using vaginal relapses and toxicities [1-8]. Considering the last aspect, different scoring systems have been applied, with different versions of the Common Terminology Criteria for Adverse Events (CTCAE) not being specifically related to radiotherapy toxicities [9-12]. Furthermore, although VCB is usually the most common brachytherapy performed in radiation oncology departments, these aspects related to fractionation schedules and toxicities does not seem to be specially analyzed considering good results obtained in local control.
Since 2004, our group has been using different HDR-VCB schedules and in 2012, we started using 3D planning brachytherapy. The results of all the schedules employed as exclusive treatment in post-operative endometrial cancer were similar in relation to local control and complications, mainly late vaginal toxicity (LVT) [7, 8]. In 2023, we compared two VCB schedules (i.e., 6 Gy × 3 vs. 7.5 Gy × 2 fractions) using dosimetry parameters, and no differences between the schedules in dosimetry parameters, VCR, and late complications were observed [7, 8]. At present, the results of external beam irradiation (EBRT) plus VCB showed differences in vaginal toxicities when two schedules were compared using diametrical parameters. In these studies, we considered dosimetry parameters useful for comparing VCB schedules to establish differences between them, and in late toxicity [7-9].
To find and examine a system comparing fractionations in VCB, the present study investigating different schedules for 3D planning was performed. Dosimetry parameters were calculated in three different computed tomography (CT) studies for three applicator diameters.
Material and methods
In this study, three 3D planning CT images corresponding to different cylinder diameters were selected from our Oncentra Brachy v. 4.6 treatment planning system. The cylinder diameters selected for analysis were 3.5 cm, 3 cm, and 2.5 cm. CT slices were reconstructed every 1 mm. Treatment technique has been described elsewhere [7]. Clinical target volume (CTV) included the top of the vagina to the end of the first cylinder to treat approximately 3 cm of the vagina with an active length of 2.5 cm (6 alternative stepping source). An active length of 4 cm (8 alternative stepping source) to treat around 4.6 cm of the vagina was also used. Doses were prescribed at 5 mm from the applicator surface, with optimization of distance based on points. The same applicator diameters and CT image data sets were employed for treatment planning with applicator surface prescription.
CTV volumes were delineated by the same clinician in the same cylinder at 3 cm and 4 cm of the vagina, and were performed for each cylinder diameter covering 3 cm or 4 cm, generating an isotropic 3 mm expansion of the cylinder volume and Boolean subtraction of the applicator. External surface of CTV was corrected in case of defect or excess using a Perl tool of 1-2 mm. Organs at risk (OARs) were outlined by a technician following the external contour. Figures 1-3 show an example of each dose distribution in axial, sagittal, and coronal planes for both prescription types, such as at the surface and at 5 mm from the applicator surface.
Fig. 1
Dose distribution using 3.5 cm applicator diameter for 2.5 cm and 4 cm active source length and dose prescription. A) Dose distribution with 2.5 cm active length and prescription at 5 mm from applicator surface in axial, coronal, and sagittal planes. B) Dose distribution with 4 cm active length and prescription at 5 mm from applicator surface in axial, coronal, and sagittal planes. C) Dose distribution with 2.5 cm active length and prescription at applicator surface in axial, coronal, and sagittal planes. D) Dose distribution with 4 cm active length and prescription at applicator surface in axial, coronal, and sagittal planes
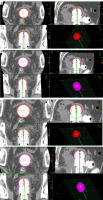
Fig. 2
Dose distribution using 3 cm applicator diameter for 2.5 cm and 4 cm active source length and dose prescription. A) 2.5 cm active length with prescription at 5 mm from applicator surface in axial, coronal, and sagittal planes. B) 4 cm active length with prescription at 5 mm from applicator surface in axial, coronal, and sagittal planes. C) 2.5 cm active length with prescription at applicator surface in axial, coronal, and sagittal planes D) 4 cm active length with prescription at applicator surface in axial, coronal, and sagittal planes
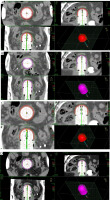
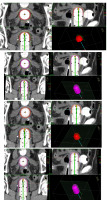
Fig. 3
Dose distribution using 2.5 cm applicator diameter for 2.5 cm and 4 cm active source length and dose prescription. A) Dose distribution with 2.5 cm active length and prescription at 5 mm from applicator surface in axial, coronal, and sagittal planes B) Dose distribution with 4 cm active length and prescription at 5 mm from applicator surface in axial, coronal, and sagittal planes. C) Dose distribution with 2.5 cm active length and prescription at applicator surface in axial, coronal, and sagittal planes. D) Dose distribution with 4 cm active length and prescription at applicator surface in axial, coronal, and sagittal planes
Tables 1 and 2 demonstrate the different fractionations selected according to the reports of the American Brachytherapy Society, the Gynae Working Group of the Groupe Européen of Curietherapy of the European Society for treatment in radiation oncology, and the Spanish BT group (GEB) of the Spanish Society of Radiation Oncology (SEOR) [2, 3, 13, 14]. Twelve fractionations were selected for active source lengths of 2.5 cm and 4 cm in order to calculate different dosimetry parameters. For each applicator diameter, fractionation schedule and active source length were calculated: prescription dose at 5 mm from the applicator, at the mucosa surface, overall dose per fractionation (Gy), vaginal surface dose per fraction (Gy), overall dose (Gy) at the vaginal surface (EQD2 α/β = 10 and α/β = 3), CTV D90 per fraction, and overall CTV D90 EQD2 (α/β = 10). Also, D2cc in OARs, such as vagina, bladder, bowel, sigmoid, and rectum in EQD2 (α/β = 3) were calculated. Statistical examination considered only a descriptive analysis of each case analyzed.
Table 1
Dosimetry parameters of clinical target volume (CTV) for cylinder diameters and active length sources considering different fractionation schedules
Table 2
Dosimetry parameters of organs at risk (OARs) for cylinder diameters and active length sources considering different fractionation schedules
Results
Tables 1 and 2 show the results of the above-mentioned dosimetry parameters. When considering an active source length of 2.5 cm, the treated vagina length was around 3 cm, while for an active source length of 4 cm, the corresponding vagina treated was about 4.6 cm. In the cases selected, CTVs for an applicator diameter of 3.5 cm with active source lengths of 2.5 cm and 4 cm were 7.9 cm3 and 12.8 cm3, respectively. The corresponding values for an applicator diameter of 3 cm were 8.0 cm3 and 11.9 cm3, respectively. For an applicator diameter of 2.5 cm, CTVs were 5.5 cm3 and 9.0 cm3.
Possibly, one of the most common fractionation schedules worldwide using HDR for exclusive post-operative VCB is 7 Gy × 3 fractions prescribed at 5 mm from the applicator surface [2, 13], with the overall surface dose of 32 Gy, 34 Gy, or 36 Gy depending on the cylinder diameter. The corresponding overall surface EQD2 (α/β = 3) for cylinder diameters of 2.5 cm, 3 cm, and 3.5 cm for an active length of 2.5 cm were 107.4 Gy, 95.1 Gy, and 88.4 Gy, respectively, with the corresponding overall surface EQD2 (α/β = 10) of 65.7 Gy, 59.2 Gy, and 55.6 Gy, respectively.
The highest overall treatment doses for the vaginal surface are administered with the schedules of 6 fractions of 4.5-5 Gy and 5 fractions of 5-5.5 Gy, all prescribed at 5 mm from the applicator, and 7.5 Gy × 5 fractions prescribed at the applicator surface. The fractionation schedule of 7 Gy × 3 fractions is in the middle range, and the remaining schedules provide a lower dose to the vaginal surface and CTV. When considering all the schedules for vaginal surface doses, these ranges are between 34 and 79.2 Gy for EQD2 (α/β = 10) and between 53 Gy and 118.3 Gy for EQD2 (α/β = 3), depending on the applicator diameter and prescription.
In the present study, the median overall D90 CTV EQD2 (α/β = 10) with a 2.5 cm active source length and applicator diameter of 2.5 cm was 35.4 Gy (range, 25.5-49.2 Gy), being 37.5 Gy for a 3 cm applicator diameter (range, 26-50 Gy), and 33.7 Gy for a 3.5 cm applicator diameter (range, 23.4-43.9 Gy). In the case of a 4 cm source length, the median D90 CTV EQD2 (α/β = 10) for a 2.5 cm applicator diameter was 36.6 Gy (range, 26.8-49.7 Gy), for an applicator diameter of 3 cm was 38.9 Gy (range, 27.1-50.3 Gy), and for an applicator diameter of 3.5 cm was 33.5 Gy (range, 23.2-43.5 Gy). Higher dose values are found for an applicator diameter of 2.5 cm, while in the present study, CTV doses were usually higher in larger active source lengths.
Figures 1-3 show the axial, sagittal, and coronal dose distributions, with applicator diameters of 3.5 cm, 3 cm, and 2.5 cm, and for 2.5 cm and 4 cm active source lengths in prescribing at 5 mm and at the applicator surface, with additional 3D view. As shown, the CTV was not adequately covered with the prescription to the surface applicator.
The dose to OAR administered in the case of 3 fractions of 7 Gy, the D2cc in bowel EQD2 (α/β = 3) was 73 Gy. In the remaining fractionation schedules, the doses to all other OARs were lower among the values considered by the Embrace-I study for late toxicities [15]. Doses in OARs were slightly higher in active source lengths of 4 cm, but not significant. On the other hand, there is a wide range when considering the D2cc of vagina and vaginal mucosa surface dose, but it is mainly related to fractionation schedule and cylinder diameter.
Discussion
When indicated, post-operative brachytherapy in endometrial cancer is a very useful treatment, with few differences among the schedules in VCRs and late complications, mainly LVT [1-3]. In 3D planning, the possible brachytherapy schedules with different applicator diameters and active source lengths have not been analyzed using dosimetry parameters, and this was the aim of the present study. To our knowledge, this is the first study in the literature describing how to evaluate and compare the most common fractionation schedules in exclusive brachytherapy of endometrial cancer. Some of the aspects found were already known from the 2D planning, and were confirmed here using 3D-based planning.
The present study reports a wide range between the vaginal surface dose and extensive variation depending on the fractionation schedule. Moreover, according to the present results, it could be hypothesized that a larger vaginal CTV receives higher doses, and larger active source lengths seem to increase the vaginal surface dose.
Although already known, taking into account the different fractionations studied globally and similar results described in different series, the present analysis indicates that an applicator of 3.5 cm should be used whenever possible in order to reduce the vaginal surface dose, which has been shown related to LVT [3, 16].
In the present analysis, the prescription to the vaginal surface did not allow for an isodose of 90% to cover the CTV, as illustrated in Figures 1-3. Therefore, with the use of this prescription, it is better to consider CTV delineation in all the cases. On the other hand, according to unpublished data from our center, the size of the vaginal wall varies from 2 to 5 mm, so the prescription to D90 CTV by graphic optimization or to the CTV external surface points (target points) should be explored in the near future. Therefore, at present, our group is performing a retrospective analysis of more than 200 patients using 3D treatment planning.
In a recent study among 110 patients using 2 different fractionation schedules (i.e., 6 Gy × 3 and 7.5 Gy × 2), there were no vaginal relapses or late complications in the rectum, bladder, or vagina. With the use of dosimetry parameters, this study showed that both schedules were similar in dose volume histogram metrics and in late toxicities [7]. LVT using objective scales for prospective assessment of treatment morbidity (LENT-SOMA) [10], scores appeared in 51/110 (46.4%) patients: 26/110 (23.6%) had telangiectasia only, and 32/110 (29%) presented vaginal stenosis: G1 in 26/110 (23.1%) (most being minimal), G2 in 5/110 (4.5%), and G3 in 1/110 (0.9%) [7]. With CTCAE v. 4 scores, telangiectasia is not considered a complication. In relation to series in the literature, there are few differences in complications, independent of the fractionation schedule. In the present study, in the different schedules analyzed, considering the differences in overall doses at the vaginal surface (α/β for 10 and 3) and the overall doses to D90 CTV (α/β = 10) and at D2cc of CTV (α/β = 3), we can hypothesize that there should be a wide difference in complications among the different schedules, although this was not observed. In this sense, taking into account our previous results and some studies in the literature, the most adequate fractionation could be with doses of less than 7 Gy × 3 fractions [7, 16]. Along this line, lower OARs doses are usually made, mainly in 2.5 cm active source length. It therefore seems that doses to the D90 CTV ≤ 30 Gy EQD2 (α/β = 10) may be sufficient to treat such patients, offering overall vaginal EQD2 (α/β = 3) doses of ≤ 80 Gy [4, 17].
The dose to D2cc in the CTV also increases with the overall dose administered. In previous retrospective studies, we found a relation of G2 LVT with D2cc EQD2 (α/β = 3) ≥ 68 Gy in the CTV of post-operative endometrial cancer patients receiving VCB ± external beam irradiation (EBRT) [18, 19]. Nevertheless, in a study using 6 Gy × 3 fractions and 7.5 Gy × 2 fractions, the need for this constraint was not shown to be necessary [7].
Doses to OARs increase with the overall dose administered and larger active source length, but there is wide variation depending on the fractionation schedule and patient anatomy. These doses are usually low, which explains the low-rate of complications in these OARs in exclusive post-operative VCB.
Another aspect to be considered was highlighted by Sikorska et al., who reported that the vaginal diameter at different levels may vary creating air bags (especially in longer CTV of 5 cm). This causes undertreatment, and plan modification should be made [20].
The main limitation of the present study is that a prospective trial in patients is needed to clarify better the most adequate doses to be administered. At present, the TOSCANE study, a 3D study analysis, is being developed by the GEB together with the Gynecological Cancer Group (GINECOR) of SEOR, in post-operative endometrial cancer patients, considering clinical and dosimetric data to evaluate the best VCB schedule. Another limitation is that in different patients, OARs may be movable and located in a slightly different position according to the vagina. Therefore, the dose results of a few cases shown in Table 2 can be considered as an estimation only. Nevertheless, in these cases, the doses in OARs are lower than the overall dose administered.
The current analysis is the first of its kind in the literature using 3D-based VCB. The study presents an analysis suggesting that overall doses lower than 30 Gy prescribed at 5 mm with an active source length of 2.5 cm and the use of an applicator of 3.5 cm provide doses to the mucosa surface of about 80 Gy EQD2 (α/β = 3). This is offered by almost all the schedules using an applicator diameter of 3.5 cm, but not in smaller applicator diameters. In fact, for a diameter of 3 cm, these values are only obtained by 5 Gy × 4 fractions, 6 Gy × 3 fractions, and 7-7.5 Gy × 2 fractions, and with even fewer possibilities using an applicator diameter of 2.5 cm for the same fractionation schedules. Moreover, it should be taken into account that the lower the number of fractions, the lesser the discomfort of the patient, the treatment costs, and a more extended use of VCB in low-access areas [16, 21, 22]. This study can help guide doctors in decision-making regarding fractionation schedules to apply in their clinical practice.
Conclusions
In conclusion, there is a wide range of fractionation schedules in the literature, which are difficult to compare. 3D planning brachytherapy provides the knowledge of real aspects of VCB in post-operative endometrial cancer. It seems that prescribing at 5 mm and the use of an applicator diameter of 3.5 cm is the most adequate for the CTV coverage with a lower mucosa dose. In relation to prescription on the surface, all the treatments should be 3D-planned in order to correctly include the CTV. Considering the present data and studies in the literature, doses less than 7 Gy × 3 fractions seem to be sufficient to treat such patients, and an active source length of 2.5 cm also seems to be enough to treat 3 cm as the CTV. All these aspects should influence the reduction of late vaginal complications. The current dosimetry study allows for better selection of fractionation schedules, and helps to unify treatments among centers. Nevertheless, prospective 3D planning trials are needed to confirm our hypotheses, and to establish the best schedule and CTV length to be used with clinical data, such as late toxicity and relapses.



